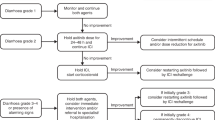
Abstract
Immune checkpoint inhibitors (ICIs) have emerged as the newest pillar of cancer treatment. Immune-mediated toxicities, stemming from increased activity within the T cell lineage, range from asymptomatic or mild complications to those that are fulminant and potentially fatal. Although they are of variable occurrence, cardiovascular, rheumatic, and renal immune-mediated toxicities are among the most serious of these adverse events. We present MASCC recommendations with respect to the workup and management of cardiovascular, rheumatic, and renal immune-mediated toxicities with a focus on presentations that require treatment with immunomodulating agents.

Similar content being viewed by others
References
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(63982):1350–1355. https://doi.org/10.1126/science.aar4060
Johnson DB, Chandra S, Sosman JA (2018) Immune checkpoint inhibitor toxicity in 2018. JAMA 320(16):1702–1703. https://doi.org/10.1001/jama.2018.13995
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4(12):1721–1728. https://doi.org/10.1001/jamaoncol.2018.3923
Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB (2018) Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391(10124):933. https://doi.org/10.1016/S0140-6736(18)30533-6
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr, Anders RA, Sosman JA, Moslehi JJ (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375(18):1749–1755. https://doi.org/10.1056/NEJMoa1609214
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG (2018) Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71(16):1755–1764. https://doi.org/10.1016/j.jacc.2018.02.037
Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, Monestier S, Grob JJ, Scemama U, Jacquier A, Lalevee N, Barraud J, Peyrol M, Laine M, Bonello L, Paganelli F, Cohen A, Barlesi F, Ederhy S, Thuny F (2017) Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 136(21):2085–2087. https://doi.org/10.1161/CIRCULATIONAHA.117.030571
Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ (2018) Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 19(12):1579–1589. https://doi.org/10.1016/S1470-2045(18)30608-9
Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T (2003) Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 9(12):1477–1483. https://doi.org/10.1038/nm955
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291(5502):319–322. https://doi.org/10.1126/science.291.5502.319.
Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH (2012) PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J Immunol 188(10):4876–4884. https://doi.org/10.4049/jimmunol.1200389
Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH (2007) Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 116(18):2062–2071. https://doi.org/10.1161/CIRCULATIONAHA.107.709360
Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH (2007) Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 117(10):2974–2982. https://doi.org/10.1172/JCI31344
Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, Jarolim P, Freeman GJ, Sharpe AH, Lichtman AH (2011) Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 31(5):1100–1107. https://doi.org/10.1161/ATVBAHA.111.224709
Reuben A, Petaccia de Macedo M, McQuade J, Joon A, Ren Z, Calderone T, Conner B, Wani K, Cooper ZA, Tawbi H, Tetzlaff MT, Padera RF, Durand JB, Lazar AJ, Wargo JA, Davies MA (2017) Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology 6(12):e1361097. https://doi.org/10.1080/2162402X.2017.1361097
Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, Guminski A, Puzanov I, Lawrence DP, Buchbinder EI, Mudigonda T, Spencer K, Bender C, Lee J, Kaufman HL, Menzies AM, Hassel JC, Mehnert JM, Sosman JA, Long GV, Clark J (2016) Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2(2):234–240. https://doi.org/10.1001/jamaoncol.2015.4368
Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV (2017) Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28(2):368–376. https://doi.org/10.1093/annonc/mdw443
Awadalla M, Golden DLA, Mahmood SS, Alvi RM, Mercaldo ND, Hassan MZO, Banerji D, Rokicki A, Mulligan C, Murphy SPT, Jones-O’Connor M, Cohen JV, Heinzerling LM, Armanious M, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Rizvi MA, Sahni G, Lyon AR, Tocchetti CG, Mercurio V, Thuny F, Ederhy S, Mahmoudi M, Lawrence DP, Groarke JD, Nohria A, Fradley MG, Reynolds KL, Neilan TG (2019) Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer 7(1):53. https://doi.org/10.1186/s40425-019-0535-y
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C (2017) Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 35(7):785–792. https://doi.org/10.1200/JCO.2015.66.1389
Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, Léonard-Louis S, Benveniste O, Moslehi JJ, Allenbach Y (2018) Immune checkpoint inhibitor-associated myositis. Circulation 138(7):743–745. https://doi.org/10.1161/CIRCULATIONAHA.118.035898
Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, Gertler AS, Moslehi JJ, Conry RM (2017) Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 5(1):91. https://doi.org/10.1186/s40425-017-0296-4
Wang DY, Okoye GD, Neilan TG, Johnson DB, Moslehi JJ (2017) Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 19(3):21. https://doi.org/10.1007/s11886-017-0835-0
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA, National Comprehensive Cancer Network (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36(17):1714–1768. https://doi.org/10.1200/JCO.2017.77.6385
Sarocchi M, Grossi F, Arboscello E, Bellodi A, Genova C, Dal Bello MG, Rijavec E, Barletta G, Rossi G, Biello F, Ghigliotti G, Canepa M, Mussap M, Brunelli C, Spallarossa P (2018) Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist 23(8):936–942. https://doi.org/10.1634/theoncologist.2017-0452
Lee Chuy K, Oikonomou EK, Postow MA, Callahan MK, Chapman PB, Shoushtari AN, Neilan TG, Fradley MG, Ramanathan LV, Wolchok JD, Steingart RM, Gupta D (2019) Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center experience. Oncologist 24(5):e196–e197. https://doi.org/10.1634/theoncologist.2019-0040
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis (2009) Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 53(17):1475–1487. https://doi.org/10.1016/j.jacc.2009.02.007
Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch-Herold M, Kwong RY (2017) Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 70(16):1964–1976. https://doi.org/10.1016/j.jacc.2017.08.050
Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G, Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology (2017) Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study. J Am Coll Cardiol 70(16):1977–1987. https://doi.org/10.1016/j.jacc.2017.08.044
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R (2007) The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 50(19):1914–1931. https://doi.org/10.1016/j.jacc.2007.09.008.
Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d’Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL (2012) 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 21(4):245–274. https://doi.org/10.1016/j.carpath.2011.10.001
Mahmood SS, Chen CL, Shapnik N, Krishnan U, Singh HS, Makker V (2018) Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: a case report. Gynecol Oncol Rep 25:74–77. https://doi.org/10.1016/j.gore.2018.05.014
Agrawal N, Khunger A, Vachhani P, Colvin TA, Hattoum A, Spangenthal E, Curtis AB, Dy GK, Ernstoff MS, Puzanov I (2019) Cardiac toxicity associated with immune checkpoint inhibitors: case series and review of the literature. Case Rep Oncol 12(1):260–276. https://doi.org/10.1159/000498985
Jain V, Mohebtash M, Rodrigo ME, Ruiz G, Atkins MB, Barac A (2018) Autoimmune myocarditis caused by immune checkpoint inhibitors treated with antithymocyte globulin. J Immunother 41(7):332–335. https://doi.org/10.1097/CJI.0000000000000239
Rota E, Varese P, Agosti S, Celli L, Ghiglione E, Pappalardo I, Zaccone G, Paglia A, Morelli N (2019) Concomitant myasthenia gravis, myositis, myocarditis and polyneuropathy, induced by immune-checkpoint inhibitors: a life-threatening continuum of neuromuscular and cardiac toxicity. ENeurologicalSci 14:4–5. https://doi.org/10.1016/j.ensci.2018.11.023
Imai R, Ono M, Nishimura N, Suzuki K, Komiyama N, Tamura T (2019) Fulminant myocarditis caused by an immune checkpoint inhibitor: a case report with pathologic findings. J Thorac Oncol 14(2):e36–e38. https://doi.org/10.1016/j.jtho.2018.10.156
Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD (2018) Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 19(11):1480–1492. https://doi.org/10.1016/S1470-2045(18)30700-9
Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, Panageas KS, Wolchok JD, Chapman PB (2018) Measuring toxic effects and time to treatment failure for nivolumab plus ipilimumab in melanoma. JAMA Oncol 4(1):98–101. https://doi.org/10.1001/jamaoncol.2017.2391
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD (2015) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33(17):1889–1894. https://doi.org/10.1200/JCO.2014.56.2736
Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, Ancell KK, Long GV, Menzies AM, Eroglu Z, Johnson DB, Shoushtari AN (2018) Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 29(1):250–255. https://doi.org/10.1093/annonc/mdx642
Abdel-Rahman O, Eltobgy M, Oweira H, Giryes A, Tekbas A, Decker M (2017) Immune-related musculoskeletal toxicities among cancer patients treated with immune checkpoint inhibitors: a systematic review. Immunotherapy 9(14):1175–1183. https://doi.org/10.2217/imt-2017-0108
Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, Korenstein D (2018) Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 360:k793. https://doi.org/10.1136/bmj.k793
Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA (2017) Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 69(11):1751–1763. https://doi.org/10.1002/acr.23177
Moseley KF, Naidoo J, Bingham CO, Carducci MA, Forde PM, Gibney GT, Lipson EJ, Shah AA, Sharfman WH, Cappelli LC (2018) Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: a seminal case series. J Immunother Cancer 6(1):104. https://doi.org/10.1186/s40425-018-0417-8
Cancer Care Ontario. Immune checkpoint inhibitor toxicity management clinical practice guideline (Version 1. 2018) [Available from: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/52976. Accessed August 30, 2019.
Abdel-Wahab N, Bingham COI, Suarez-Almazor ME (2019) Musculoskeletal and rheumatologic toxicities. In: Earstoff MS, Puzanov I, Robert C, Daib A, Hersey P (eds) SITC’s Guide to Managing Immunotherapy Toxicity. Demos Medical Publishing - Springer Publishing, New York, pp 171–184
Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, ESMO Guidelines Committee (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv119–iv142. https://doi.org/10.1093/annonc/mdx225
Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 5(1):95. https://doi.org/10.1186/s40425-017-0300-z
Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, Ernstoff MS, Frigault M, Hoffner B, Hoimes CJ, Lacouture M, Locke F, Lunning M, Mohindra NA, Naidoo J, Olszanski AJ, Oluwole O, Patel SP, Reddy S, Ryder M, Santomasso B, Shofer S, Sosman JA, Wahidi M, Wang Y, Johnson-Chilla A, Scavone JL (2019) Management of immunotherapy-related toxicities, Version 1.2019. J Natl Compr Cancer Netw 17(3):255–289. https://doi.org/10.6004/jnccn.2019.0013
Abdel-Wahab N, Shah M, Suarez-Almazor ME (2016) Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 11(7):e0160221. https://doi.org/10.1371/journal.pone.0160221
Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, Ederhy S, Feuillet S, François H, Lazarovici J, Le Pavec J, De Martin E, Mateus C, Michot JM, Samuel D, Soria JC, Robert C, Eggermont A, Marabelle A (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27(4):559–574. https://doi.org/10.1093/annonc/mdv623
Buder-Bakhaya K, Benesova K, Schulz C, Anwar H, Dimitrakopoulou-Strauss A, Weber TF, Enk A, Lorenz HM, Hassel JC (2018) Characterization of arthralgia induced by PD-1 antibody treatment in patients with metastasized cutaneous malignancies. Cancer Immunol Immunother 67(2):175–182. https://doi.org/10.1007/s00262-017-2069-9
Le Burel S, Champiat S, Mateus C, Marabelle A, Michot JM, Robert C, Belkhir R, Soria JC, Laghouati S, Voisin AL, Fain O, Mékinian A, Coutte L, Szwebel TA, Dunogeant L, Lioger B, Luxembourger C, Mariette X, Lambotte O (2017) Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer 82:34–44. https://doi.org/10.1016/j.ejca.2017.05.032
Lidar M, Giat E, Garelick D, Horowitz Y, Amital H, Steinberg-Silman Y, Schachter J, Shapira-Frommer R, Markel G (2018) Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 17(3):284–289. https://doi.org/10.1016/j.autrev.2018.01.003
Richter MD, Crowson C, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U (2019) Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheum 71(3):468–475. https://doi.org/10.1002/art.40745
Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, Dousset L, Pham-Ledard A, Prey S, Beylot-Barry M, Daste A, Gross-Goupil M, Lallier J, Ravaud A, Forcade E, Bannwarth B, Truchetet ME, Richez C, Mehsen N, Schaeverbeke T, FHU ACRONIM (2018) Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 77(3):393–398. https://doi.org/10.1136/annrheumdis-2017-212257
Calabrese C, Kirchner E, Kontzias K, Velcheti V, Calabrese LH (2017) Rheumatic immune-related adverse events of checkpoint therapy for cancer: case series of a new nosological entity. RMD Open 3(1):e000412. https://doi.org/10.1136/rmdopen-2016-000412
Belkhir R, Burel SL, Dunogeant L, Marabelle A, Hollebecque A, Besse B, Leary A, Voisin AL, Pontoizeau C, Coutte L, Pertuiset E, Mouterde G, Fain O, Lambotte O, Mariette X (2017) Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 76(10):1747–1750. https://doi.org/10.1136/annrheumdis-2017-211216
Kuswanto WF, MacFarlane LA, Gedmintas L, Mulloy A, Choueiri TK, Bermas BL (2018) Rheumatologic symptoms in oncologic patients on PD-1 inhibitors. Semin Arthritis Rheum 47(6):907–910. https://doi.org/10.1016/j.semarthrit.2017.10.018
Mitchell EL, Lau PK, Khoo C, Liew D, Leung J, Liu B et al (2018) Management of rheumatological immune related adverse events in melanoma patients treated with anti-programmed cell death (PD) 1 antibodies. Pigment Cell Melanoma Res 31(1):190. https://doi.org/10.1111/pcmr.12656
Abdel-Wahab N, Suarez-Almazor ME (2019) Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology (Oxford) 58(Suppl 7):vii40–vii48. https://doi.org/10.1093/rheumatology/kez297
Abdel-Wahab N, Tayar JH, Diab A, Kim ST, Lu H, Suarez-Almazor ME (2017) Inflammatory arthritis induced by the use of checkpoint inhibitors for immunotherapy of cancer. J Immunother Cancer 5(2):215
Pundole X, Abdel-Wahab N, Suarez-Almazor ME (2019) Arthritis risk with immune checkpoint inhibitor therapy for cancer. Curr Opin Rheumatol 31(3):293–299. https://doi.org/10.1097/BOR.0000000000000601
Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, Zheng L, Bingham CO 3rd, Shah AA (2018) Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum 48(3):553–557. https://doi.org/10.1016/j.semarthrit.2018.02.011
Cappelli LC, Gutierrez AK, Baer AN, Albayda J, Manno RL, Haque U, Lipson EJ, Bleich KB, Shah AA, Naidoo J, Brahmer JR, Le D, Bingham CO 3rd (2017) Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 76(1):43–50. https://doi.org/10.1136/annrheumdis-2016-209595
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588. https://doi.org/10.1136/ard.2010.138461
Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L (2019) Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open 5(1):e000906. https://doi.org/10.1136/rmdopen-2019-000906
Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, Bachta A, Dejaco C, Duftner C, Jensen HS, Duhaut P, Poór G, Kaposi NP, Mandl P, Balint PV, Schmidt Z, Iagnocco A, Nannini C, Cantini F, Macchioni P, Pipitone N, Amo MD, Espígol-Frigolé G, Cid MC, Martínez-Taboada VM, Nordborg E, Direskeneli H, Aydin SZ, Ahmed K, Hazleman B, Silverman B, Pease C, Wakefield RJ, Luqmani R, Abril A, Michet CJ, Marcus R, Gonter NJ, Maz M, Carter RE, Crowson CS, Matteson EL (2012) 2012 Provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 71(4):484–492. https://doi.org/10.1136/annrheumdis-2011-200329
Daxini A, Cronin K, Sreih AG (2018) Vasculitis associated with immune checkpoint inhibitors-a systematic review. Clin Rheumatol 37(9):2579–2584. https://doi.org/10.1007/s10067-018-4177-0
Goldstein BL, Gedmintas L, Todd DJ (2014) Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheum 66(3):768–769. https://doi.org/10.1002/art.38282
Pundole X, Shah M, Abdel-Wahab N, Suarez-Almazor EM (2017) Immune checkpoint inhibitors and inflammatory myopathies: data from the US Food and Drug Administration Adverse Event Reporting System. Arthritis Rheum 69(10):1192–1193
Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME (2019) Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum 48(4):736–740. https://doi.org/10.1016/j.semarthrit.2018.05.006
Liewluck T, Kao JC, Mauermann ML (2018) PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother 41(4):208–211. https://doi.org/10.1097/CJI.0000000000000196
Moreira A, Loquai C, Pfohler C, Kahler KC, Knauss S, Heppt MV, Gutzmer R, Dimitriou F, Meier F, Mitzel-Rink H, Schuler G, Terheyden P, Thoms KM, Türk M, Dummer R, Zimmer L, Schröder R, Heinzerling L (2019) Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors. Eur J Cancer 106:12–23. https://doi.org/10.1016/j.ejca.2018.09.033
Kang KH, Grubb W, Sawlani K, Gibson MK, Hoimes CJ, Rogers LR, Lavertu P, Yao M (2018) Immune checkpoint-mediated myositis and myasthenia gravis: a case report and review of evaluation and management. Am J Otolaryngol 39(5):642–645. https://doi.org/10.1016/j.amjoto.2018.06.003
Mohn N, Suhs KW, Gingele S, Angela Y, Stangel M, Gutzmer R, Satzger I, Skripuletz T (2019) Acute progressive neuropathy-myositis-myasthenia-like syndrome associated with immune-checkpoint inhibitor therapy in patients with metastatic melanoma. Melanoma Res 29(4):435–440. https://doi.org/10.1097/CMR.0000000000000598
Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, Uhara H, Hasegawa Y, Inomata S, Otani Y, Yokota K, Hirose T, Tanaka R, Suzuki N, Matsui M (2017) Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 89(11):1127–1134. https://doi.org/10.1212/WNL.0000000000004359
Starke A, Lindenmeyer MT, Segerer S, Neusser MA, Rusi B, Schmid DM, Cohen CD, Wüthrich RP, Fehr T, Waeckerle-Men Y (2010) Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int 78(1):38–47. https://doi.org/10.1038/ki.2010.97
Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, Le DT, Lipson EJ, Glezerman IG, Wolchok J, Cornell LD, Feldman P, Stokes MB, Zapata SA, Hodi FS, Ott PA, Yamashita M, Leaf DE (2016) Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90(3):638–647. https://doi.org/10.1016/j.kint.2016.04.008
Abdel-Rahman O, Fouad M (2016) A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy. 8(5):665–674. https://doi.org/10.2217/imt-2015-0020
Shirali AC, Perazella MA, Gettinger S (2016) Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am J Kidney Dis 68(2):287–291. https://doi.org/10.1053/j.ajkd.2016.02.057
Izzedine H, Mathian A, Champiat S, Picard C, Mateus C, Routier E, Varga A, Malka D, Leary A, Michels J, Michot JM, Marabelle A, Lambotte O, Amoura Z, Soria JC, Kaaki S, Quellard N, Goujon JM, Brocheriou I (2019) Renal toxicities associated with pembrolizumab. Clin Kidney J 12(1):81–88. https://doi.org/10.1093/ckj/sfy100
Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A (2019) Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 7(1):2. https://doi.org/10.1186/s40425-018-0478-8
Glezerman IG, Latcha S, Jaimes EA (2018) Renal immune related adverse events in patients treated with PD-1 inhibitors: an emerging complication of immunotherapy. J Am Soc Nephrol 29:44
Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, Murakami N, Herrmann SM, Manohar S, Shirali AC, Kitchlu A, Shirazian S, Assal A, Vijayan A, Renaghan AD, Ortiz-Melo DI, Rangarajan S, Malik AB, Hogan JJ, Dinh AR, Shin DS, Marrone KA, Mithani Z, Johnson DB, Hosseini A, Uprety D, Sharma S, Gupta S, Reynolds KL, Sise ME, Leaf DE (2020) Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol 31(2):435–446. https://doi.org/10.1681/ASN.2019070676
Acknowledgments
Professor BL Rapoport is supported by supported by the Cancer Association of South Africa (CANSA) and the National Research Foundation (NRF) of South Africa. Dr. I. Glezerman is supported by the NIH/NCI (Cancer Center Support Grant P30 CA008748).
Author information
Authors and Affiliations
Contributions
All of the authors contributed equally to the conceptualization of the manuscript; MES-A, XP, NA-W, DBJ, IG, and TC shared sections on rheumatic immune-related adverse events equally, while BLR and DBJ provided clinical input and BLR, DBJ, and RA editorial oversight. All of the authors provided critical appraisal of the manuscript and approve of its submission.
Corresponding author
Ethics declarations
Conflict of interest
AW, XP, AB, RA, JC, TC, PG, DG RAG, and VRS have no conflict of interest to declare. MD reports grants from Novartis, other (SAB) from Neoleukin Therapeutics, personal fees from Partner Therapeutics, personal fees from Tillotts Pharma, and grants from Genentech, outside the submitted work. MG reports consultant work with Bristol Myers Squibb (BMS), and AstraZeneca, outside the submitted work. IG reports other (Stock Ownership) from Pfizer Inc., and personal fees from CytomX Inc., outside the submitted work. DBJ reports other (advisory board) from Array Biopharma, grants and other (advisory board) from BMS, other (advisory board) from Jansen, grants from Incyte, other (advisory board) from Merck, and other (advisory board) from Novartis, outside the submitted work. In addition, DBJ has a patent co-inventor on use of CTLA-4 agonist for IAEs pending. BLR reports personal fees and other (advisory board) from Merck and Co.; grants, personal fees, and other (advisory board) from BMS; grants, personal fees, and other (advisory board) from Roche South Africa; and personal fees and other (advisory board) from AstraZeneca, during the conduct of the study. MSA reports personal fees from Gilead, grants from Pfizer, and personal fees from Abbvie, outside the submitted work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suarez-Almazor, M.E., Pundole, X., Abdel-Wahab, N. et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune-mediated cardiovascular, rheumatic, and renal toxicities from checkpoint inhibitors. Support Care Cancer 28, 6159–6173 (2020). https://doi.org/10.1007/s00520-020-05710-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05710-8