Abstract
Introduction
Patient-reported outcomes (PROs) have been widely accepted in western countries. However, limited attention has been given to PROs in China due to a lack of research on the agreement between doctors’ and patients’ reports of adverse events. This study aims to reveal the perception gap of chemotherapy-induced adverse events between doctors and cancer patients in China.
Methods
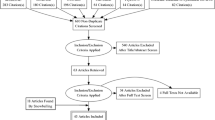
An observational study was administered at Sun Yat-Sen University Cancer Center (SYSUCC). Totally, 200 adult cancer patients undergoing chemotherapy participated. Patient reports were collected by nurses via telephone. Doctor reports were collected by nurses based on their medical records. The agreement between doctors and patients was analyzed by Cohen’s κ.
Results
Agreement between doctors and patients varied among different symptoms: 0.26 for nausea/vomiting, 0.49 for constipation, 0.63 for diarrhea, 0.65 for general pain, and 0.76 for rash. Doctors’ underreporting rates were 70% for nausea/vomiting, 50% for diarrhea, 38% for rash, 33% for constipation, and 29% for general pain.
Conclusions
The perception gap of chemotherapy-induced adverse events between doctors and patients exists in China, especially regarding subjective symptoms. Introduction of PROs in both clinical trials and routine clinical practice should be considered in China.

Similar content being viewed by others
References
Di Maio M, Basch E, Bryce J, Perrone F (2016) Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol 13:319
Carnio S, Galetta D, Scotti V, Cortinovis D, Antonuzzo A, Pisconti S, Rossi A, Martelli O, Cecere F, Lunghi A (2018) Chemotherapy-induced nausea and vomiting (CINV) in patients with advanced lung cancer during the first-line treatment: assessment by physicians, nurses, and patients from an Italian multicenter survey. Support Care Cancer 26:1841–1849
Di Maio M, Gallo C, Leighl NB, Piccirillo MC, Daniele G, Nuzzo F, Gridelli C, Gebbia V, Ciardiello F, De Placido S (2015) Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials
Gravis G, Marino P, Joly F, Oudard S, Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B (2014) Patients’ self-assessment versus investigators’ evaluation in a phase III trial in non-castrate metastatic prostate cancer (GETUG-AFU 15). Eur J Cancer 50:953–962
Warsame R, D’Souza A (2019) Patient reported outcomes have arrived: a practical overview for clinicians in using patient reported outcomes in oncology. In: Editor (ed)^(eds) Book Patient Reported Outcomes Have Arrived: A Practical Overview for Clinicians in Using Patient Reported Outcomes in Oncology. Elsevier, City
Vodicka E, Kim K, Devine E, Gnanasakthy A, Scoggins J, Patrick D (2015) Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007–2013). Contemporary Clinical Trials 43:1–9
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP (2014) Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106:dju244
Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho A, Holden J (2017) International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open 7:e017902
Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM, O’Mara AM (2015) Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncology 1:1051–1059
Bennett AV, Dueck AC, Mitchell SA, Mendoza TR, Reeve BB, Atkinson TM, Castro KM, Denicoff A, Rogak LJ, Harness JK (2016) Mode equivalence and acceptability of tablet computer-, interactive voice response system-, and paper-based administration of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Health Qual Life Outcomes 14:24
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data biometrics: 159-174
Basch E, Dueck AC, Rogak LJ, Mitchell SA, Minasian LM, Denicoff AM, Wind JK, Shaw MC, Heon N, Shi Q (2018) Feasibility of implementing the patient-reported outcomes version of the common terminology criteria for adverse events in a multicenter trial: NCCTG N1048. J Clin Oncol 36:3120
Yeung AR, Pugh SL, Klopp AH, Gil KM, Wenzel L, Westin SN, Gaffney DK, Small W Jr, Thompson S, Doncals DE (2020) Improvement in patient-reported outcomes with intensity-modulated radiotherapy (RT) compared with standard RT: a report from the NRG Oncology RTOG 1203 Study. J Clin Oncol 38:1685–1692
Basch E, Abernethy AP, Mullins CD, Reeve BB, Smith ML, Coons SJ, Sloan J, Wenzel K, Chauhan C, Eppard W (2012) Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 30:4249–4255
Dueck AC, Scher HI, Bennett AV, Mazza GL, Thanarajasingam G, Schwab G, Weitzman AL, Rogak LJ, Basch E (2020) Assessment of adverse events from the patient perspective in a phase 3 metastatic castration-resistant prostate cancer clinical trial. JAMA Oncology 6:e193332
Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. The Lancet Oncology 7:903–909
Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB (2016) Chemotherapy-induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain 157:560–568
Mols F, Beijers T, Lemmens V, van den Hurk CJ, Vreugdenhil G, van de Poll-Franse LV (2013) Chemotherapy-induced neuropathy and its association with quality of life among 2-to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 31:2699–2707
Fromme EK, Eilers KM, Mori M, Hsieh Y-C, Beer TM (2004) How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol 22:3485–3490
Montemurro F, Mittica G, Cagnazzo C, Longo V, Berchialla P, Solinas G, Culotta P, Martinello R, Foresto M, Gallizioli S (2016) Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncology 2:445–452
Sun J, Lin Q, Zhao P, Zhang Q, Xu K, Chen H, Hu CJ, Stuntz M, Li H, Liu Y (2017) Reducing waiting time and raising outpatient satisfaction in a Chinese public tertiary general hospital-an interrupted time series study. BMC Public Health 17:668
Cirillo M, Venturini M, Ciccarelli L, Coati F, Bortolami O, Verlato G (2009) Clinician versus nurse symptom reporting using the National Cancer Institute—common terminology criteria for adverse events during chemotherapy: results of a comparison based on patient's self-reported questionnaire. Ann Oncol 20:1929–1935
García M, Rohlfs I, Vila J, Sala J, Pena A, Masiá R, Marrugat J (2005) Comparison between telephone and self-administration of Short Form Health Survey Questionnaire (SF-36). Gac Sanit 19:433–439
Magnus BE, Liu Y, He J, Quinn H, Thissen D, Gross HE, DeWalt DA, Reeve BB (2016) Mode effects between computer self-administration and telephone interviewer-administration of the PROMIS® pediatric measures, self-and proxy report. Qual Life Res 25:1655–1665
Maisto SA, Conigliaro JC, Gordon AJ, McGinnis KA, Justice AC (2008) An experimental study of the agreement of self-administration and telephone administration of the Timeline Followback interview. Journal of Studies on Alcohol and Drugs 69:468–471
Feldman SF, Lapidus N, Cosnes J, Tiret E, Fonquernie L, Cabane J, Chazouilleres O, Surgers L, Beaussier M, Valleron A-J (2017) Comparing inpatient satisfaction collected via a web-based questionnaire self-completion and through a telephone interview: an ancillary study of the SENTIPAT randomized controlled trial. J Med Internet Res 19:e293
Acknowledgments
We would like to thank all the participating patients, their families, investigators, clinical research coordinators, and study nurses.
Funding
The study was supported by the following funds: (1) Science and Technology Program of Guangzhou (Grant No. 201704020072), (2) the National Key R&D Program of China (Grant No. 2016YFC0905500, 2016YFC0905503), and (3) Medical Scientific Research Foundation of Guangdong Province (Grant No. C2018062 and A2020153).
Author information
Authors and Affiliations
Contributions
LZ, RG, JY, ZZ, XL, and HZ contributed to the study design, data collection, data interpretation, and drafting of the manuscript. LZ and RG supervised the study. JY, ZZ, XL, HZ, YH, WF, YY, and SH contributed to the data collection and management. All the authors were involved in the provision of study materials and patients and data interpretation. All the authors contributed to the writing and critical review of the manuscript. JY, ZZ, XL, and HZ contributed equally to the article.
Corresponding authors
Ethics declarations
Disclaimer
The funding organizations had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Zhang, Z., Zhou, H. et al. The perception gap of chemotherapy-induced adverse events between doctors and cancer patients: an observational study in China. Support Care Cancer 29, 1543–1548 (2021). https://doi.org/10.1007/s00520-020-05649-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05649-w