Abstract
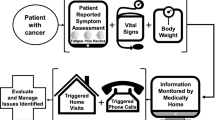
Homcology is a project that represents both an opportunity for patients who may benefit from chemotherapy so far, but present physical and social problems that prevent day-hospital access, and a model of “no-profit” contribution to the Public Health System. Our medical oncology department conducted the project from May 2014 to January 2019. We included frail patients (G-8 < 14), with advanced disease, treated with oral, subcutaneous, or parenteral biological agents, with limitations to day-hospital access, comorbidities, and at least 6-month life expectancy. A multidisciplinary team included three oncologists, four nurses, an anesthetist, a psychologist, and a physiotherapist. Satisfaction was evaluated with FAMCARE scale. A total of 188 patients (median age of 73 years, 38–87) were enrolled. Ninety percent of patients presented with metastatic disease and a median G-8 score of 8.8 (3–13.5). All of them received anticancer treatment and concomitant supportive care; 24 patients received two or more lines of treatment. The median duration of taking care was 175 days (7–1200). A median number of 254 (195–325) nursing and 164 (139–190) medical visits were performed a year, with an average of 1.9 and 1.2 visits a month per patient respectively. The median number of in-line patients was 20 (17–25). Hospitalization occurred in 18% of cases. One-third of them died at home. The others were referred to hospice. Our experience shows that the integration of home cancer treatment and supportive care is effective. Hospitalization rate is lower than data reported in the literature. Results need to be confirmed in prospective pharmacoeconomics studies.



Similar content being viewed by others
References
Jemal A, Ward E, Thun M (2010) Declining death rates reflect progress against cancer. PLoS One 5(3):e9584
Bordonaro S, Romano F, Lanteri E (2014) Effect of a structured, active, home-based cancer-treatment program for the management of patients on oral chemotherapy. Patient Prefer Adherence 8:917–923
Pernas S, Tolaney SM, Winer EP, Goel S (2018) CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol 10:1–15
Zhong H, Chan G, Hu Y, Hu H, Ouyang D (2018) A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics 10:263
Payne SA (1992) A study of quality of life in cancer patients receiving palliative chemotherapy. Soc Sci Med 35(12):1505–1509
O’Neill VJ, Twelves CJ (2002) Oral cancer treatment: developments in chemotherapy and beyond. Br J Cancer 87:933–937
De Portu S, Mantovani LG, Ravaioli A et al (2010) Cost analysis of capecitabine vs 5-fluorouracil-based treatment for metastatic colorectal cancer patients. J Chemother 22(2):125–128
Cammà C, Cabibbo G, Petta S, WEF study group; SOFIA study group et al (2013) Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology 57(3):1046–1054
Conte PF, Giovannelli S (2005) Oral vinorelbine in breast cancer. Breast Cancer Res 7:S25
Twelves C, Wong A, Nowacki MP, Abt M, Burris H, Carrato A et al (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352:2696–2704
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, de Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK (2013) Pazopanib versus Sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731
Khalaf DJ, Annala M, Taavitsainen S, Finch DL, Oja C, Vergidis J, Zulfiqar M, Sunderland K, Azad AA, Kollmannsberger CK, Eigl BJ, Noonan K, Wadhwa D, Attwell A, Keith B, Ellard SL, le L, Gleave ME, Wyatt AW, Chi KN (2019) Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label phase 2, cross-over trial. Lancer Oncol 20:1730–1739
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee H et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113–125
Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, Chiarion Sileni V, Schachter J, Garbe C, Bondarenko I, Gogas H, Mandalá M, Haanen JBAG, Lebbé C, Mackiewicz A, Rutkowski P, Nathan PD, Ribas A, Davies MA, Flaherty KT, Burgess P, Tan M, Gasal E, Voi M, Schadendorf D, Long GV (2019) Five-year outcome with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626–636
Liu G, Franssen E, Fitch MI, Warner E (1997) Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15:110–115
Freytes CO (2000) Indications and complications of intravenous devices for chemotherapy. Curr Opin Oncol 12(4):303–307
Partridge AH, Avorn J, Wang PS, Winer EP (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94(9):652–661
Tralongo P, Ferraù F, Borsellino N, Verderame F, Caruso M, Giuffrida D, Butera A, Gebbia V (2011) Cancer patient-centered home care: a new model for health care in oncology. Ther Clin Risk Manag 7:387–392
Rischin D, White MA, Matthews JP, Toner GC, Watty K, Sukowski AJ et al (2000) A randomized crossover trial of chemotherapy in the home: patient preferences and cost analysis. Med J Aust 173:125–127
Bellera C, Rainfray M, Mathoulin-Pélissier S et al (2012) Screening older cancer patients: first evaluation of the G-8 screening tool. Ann Oncol 23:2166–2172
Sewtz C, Muscheites W, Kriesen U et al (2018) Questionnaires measuring quality of life and satisfaction of patients and their relatives in a palliative care setting-German translation of FAMCARE-2 and the palliative care subscale of FACIT-Pal. Ann Palliat Med 2018 Oct 7(4):420–426
World Health Organization (1980) WHO handbook for reporting results of cancer treatment. Neoplasma 20:37–46
George J, Shalansky SJ (2007) Predictors of refill non-adherence in patients with heart failure. Br J Clin Pharmacol 63:488–493
Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, Johnson JA (2006) A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 333:15–20
Weingart SN, Brown E, Bach PB et al (2008) NCCN task force report: oral chemotherapy. J Natl Compr Cancer Netw 6(Suppl 3):1–14
Bedell CH (2003) A changing paradigm for cancer treatment: the advent of new oral chemotherapy agents. Clin J Oncol Nurs 7(Suppl 6):5–9
Raiti SBF, Di Mari A et al (2012) Active home-based cancer treatment. J Multidiscip Healthc 5:137–143
Bedell CH (2003) A changing paradigm for cancer treatment: the advent of oral chemotherapy agents. Clin J Oncol Nurs 7(Suppl 6):5–9
Funding
This project was actively funded by a not-for-profit organization, “Varese per l’Oncologi” ONLUS.
Author information
Authors and Affiliations
Contributions
Contribution: C.C. designed the study; C.C., L.B., E.M., and A. G. gathered data; C.C., I.P., M.S., and O.N. drafted the article; and G.T. and G.P. revised it critically for important intellectual content. All authors have approved the submitted version of this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chini, C., Bascialla, L., Giaquinto, A. et al. Homcology: home chemotherapy delivery in a simultaneous care project for frail advanced cancer patients. Support Care Cancer 29, 917–923 (2021). https://doi.org/10.1007/s00520-020-05569-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-020-05569-9