Summary
Aim
To assess the adherence to treatment, sustained virologic response (SVR) rate, and reinfection rate in hepatitis C patients with and without intravenous drug use.
Methods
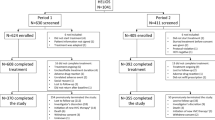
This retrospective study included hepatitis C patients, evaluated and treated in our hepatology outpatient clinic between January 2014 and October 2019.
The following information was extracted from the patient’s file: the presence of positive viral load for hepatitis C virus (HCV), active and recent (in the last 6 months) use of i.v. drugs, HCV genotype, treatment regimen, SVR, HCV reinfection rate, coinfection with human immunodeficiency virus (HIV) and ongoing opioid substitution therapy (OST).
Results
We included 431 hepatitis C patients, 234 people who inject drugs (PWID) and 197 non-PWID. Most patients were treated with direct-acting antivirals (DAA) only.
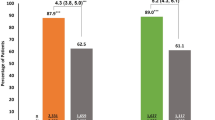
The rate of documented SVR by treated patients was significantly higher in the non-PWID cohort (91.5% vs. 61.5%, p < 0.0001), while noncompliance (did not show up to start treatment) rate or refusal of treatment was significantly higher in the PWID cohort (19.4% vs. 8.9%, p = 0.004).
In the PWID cohort, younger age and recent (in the last 6 months) or ongoing i.v. drug use was associated with noncompliance: 31.1 ± 8.4 years vs. 35.8 ± 10.6 years (p = 0.02) and 33.3% vs. 12.8% (p = 0.0008), respectively.
Ongoing OST was associated with better compliance: 61.1% vs. 46.1% (p = 0.04).
Conclusion
To achieve elimination of hepatitis C better treatment strategies are needed, especially in PWIDs.
Similar content being viewed by others
Abbreviations
- DAA:
-
Direct-acting antivirals
- EOT:
-
End of treatment
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- Non-PWID:
-
People who do not inject drugs
- OST:
-
Opioid substitution therapy
- PegIFN:
-
Pegylated interferon
- PWID:
-
People who inject drugs
- SVR:
-
Sustained virologic response
- WHO:
-
World Health Organization
References
WHO. Global surveillance and control of Hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Liver. 1999;6:34–5.
https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed: 12. October 2020.
Kandeel A, Genedy M, El-Refai S, et al. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017;37:45–53.
Gheorghe L, Csiki IE, Iacob S, et al. The prevalence and risk factors of hepatitis C virus infection in adult population in Romania: a nationwide survey 2006–2008. J Gastrointestin Liver Dis. 2010;19:373–9.
https://cdafound.org/dashboard/polaris/maps_prev.html. Accessed 28 Mar 2020.
The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–76.
Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506–10.
Chemello L, Cavalletto L, Bernardinello E, et al. The effect of interferon alfa and ribavirin combination therapy in naive patients with chronic hepatitis C. J Hepatol. 1995;23(Suppl 2):8–12.
Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–65.
Marcellin P, Cheinquer H, Curescu M, et al. High sustained virologic response rates in rapid virologic response patients in the large real-world PROPHESYS cohort confirm results from randomized clinical trials. Hepatology. 2012;56:2039–50.
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–893.
Afdhal N, Zeuzem S, Kwo P, et al. ION‑1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98.
Toyoda H, Atsukawa M, Watanabe T, et al. Real-world experience of 12-week direct-acting antiviral regimen of glecaprevir and pibrentasvir in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2019; https://doi.org/10.1111/jgh.14874.
Buggisch P, Wursthorn K, Stoehr A, et al. Real-world effectiveness and safety of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir hepatitis C treatment in a single centre in Germany. PLoS ONE. 2019;14:e214795.
World Health Organization. Global health sector strategy on viral hepatitis, 2016–2021. Geneva: World Health Organization; 2016.
Heffernan A, Cooke GS, Nayagam S, et al. Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet. 2019;393:1319–29.
Pedrana A, Howell J, Schröder S, et al. Eliminating viral hepatitis: the investment case. Doha: World Innovation Summit for Health; 2018.
https://cdafound.org/dashboard/polaris/maps.html#pablo. Accessed 28 Mar 2020.
Burnet Institute. The Prime study: treating hepatitis C in primary healthcare setting. 2018. www.burnet.edu.au. Accessed: 12. October 2020.
Litwin AH, Agyemang L, Akiyama M, et al. The PREVAIL Study: Intensive models of HCV care for people who inject drugs. J Hepatol. 2017;66(Supplement):72.
Schmidbauer C, Schuetz A, Schwanke C, et al. Interim results of an ongoing project to eliminate chronic hepatitis C in people who inject drugs with ongoing intravenous drug use and a high risk of non-adherence to direct-acting antivirals in Vienna. J Hepatol. 2019;70:e239.
Alimohammadi A, Holeksa J, Thiam A, et al. Real-world efficacy of direct-acting antiviral therapy for HCV infection affecting people who inject drugs delivered in a multidisciplinary setting. Open Forum Infect Dis. 2018;5:ofy120.
Lampertico P, Carrión JA, Curry M, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: a meta-analysis. J Hepatol. 2020; https://doi.org/10.1016/j.jhep.2020.01.025.
Rossi C, Butt ZA, Wong S, et al. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol. 2018;69:1007–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Peck-Radosavljevic declares consultation and speaker fees from AbbVie, Gilead, MSD. S. Bota: declares speaker fees from AbbVie. F. Hucke, C. Urak, K. Flatscher and M. Razpotnik declare that they have no competing interests.
Ethical standards
This study was approved by the ethics committee of our institution (approval number A38/18), and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bota, S., Razpotnik, M., Hucke, F. et al. Challenges in hepatitis C elimination despite highly effective antiviral agents in patients with and without intravenous drug use. Wien Klin Wochenschr 133, 641–646 (2021). https://doi.org/10.1007/s00508-021-01868-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-021-01868-1