Abstract
Background
CD19-specific chimeric antigen receptor (CAR) T-cell therapy has shown promising disease responses in patients with high-risk B-cell malignancies. However, its use may be related to complications such as immune-mediated complications, infections, and end-organ dysfunction. The incidence of post-CAR T-cell therapy acute kidney injury (AKI) in the children, adolescent, and young adult (CAYA) patient population is largely unreported.
Methods
The objectives of this study were to determine the incidence of AKI in CAYA patients with high-risk B-cell malignancies treated with CD19-CAR T-cell therapy, evaluate potential risk factors for developing AKI, and determine patterns of kidney function recovery. We conducted a retrospective analysis of 34 CAYA patients treated with CD19-CAR T-cell at a single institution.
Results
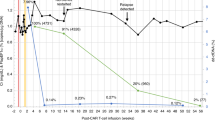
There was a cumulative incidence of any grade AKI by day 30 post-infusion of 20% (n = 7), with four cases being severe AKI (stages 2–3) and one patient requiring kidney replacement therapy. All episodes of AKI developed within the first 14 days after receiving CAR T-cell therapy and 50% of patients with AKI recovered kidney function to baseline within 30 days post-infusion. No evaluated pre-treatment risk factors were associated with the development of subsequent AKI; there was an association between AKI and cytokine release syndrome and neurotoxicity. We conclude that the risk of developing AKI following CD19-CAR T-cell therapy is highest early post-infusion, with most cases of AKI being severe.
Conclusions
Frequent monitoring to facilitate early recognition and subsequent management of kidney complications after CD19-CAR T-cell therapy may reduce the severity of AKI in the CAYA patient population.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (AT) upon reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- AlloHCT:
-
Allogeneic hematopoietic cell transplantation
- ASTCT:
-
American Society of Transplantation and Cellular Therapy
- B-ALL:
-
B-cell acute lymphoblastic leukemia
- CAYA:
-
Children, adolescent, and young adult
- CAR:
-
Chimeric antigen receptor
- CRS:
-
Cytokine release syndrome
- eGFR:
-
Estimated glomerular filtration rate
- GFR:
-
Glomerular filtration rate
- ICANS:
-
Immune effector cell-associated neurotoxicity syndrome
- KDIGO:
-
Kidney Disease: Improving Global Outcomes
- NTX:
-
Neurotoxicity
- sCr:
-
Serum creatinine
References
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507–1517
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H et al (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378:439–448
Hayden PJ, Roddie C, Bader P, Basak GW, Bonig H, Bonini C et al (2022) Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol 33:259–275
Talleur AC, Myers R, Annesley C, Shalabi H (2022) Chimeric antigen receptor T-cell therapy: current status and clinical outcomes in pediatric hematologic malignancies. Hematol Oncol Clin North Am 36:701–727
Stefanski H, Eaton A, Baggott C, Rossoff J, Verneris MR, Keating AK et al (2023) Higher doses of tisagenlecleucel associate with improved outcomes: a report from the pediatric real-world CAR consortium. Blood Adv 7:541–548
Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ et al (2017) Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med 45:e124–e131
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN et al (2019) ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625–638
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö et al (2017) Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 377:2545–2554
Gupta S, Seethapathy H, Strohbehn IA, Frigault MJ, O’Donnell EK, Jacobson CA et al (2020) Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis 76:63–71
Kizilbash SJ, Kashtan CE, Chavers BM, Cao Q, Smith AR (2016) Acute kidney injury and the risk of mortality in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22:1264–1270
Lee MD, Strohbehn IA, Seethapathy HS, Rusibamayila N, Casey KS, Gupta S et al (2021) Acute kidney injury after the CAR-T therapy tisagenlecleucel. Am J Kidney Dis 77:990–992
Farooqui N, Sy-Go JPT, Miao J, Mehta R, Vaughan LE, Bennani NN et al (2022) Incidence and risk factors for acute kidney injury after chimeric antigen receptor T-cell therapy. Mayo Clin Proc 97:1294–1304
Jhaveri KD, Rosner MH (2018) Chimeric antigen receptor T cell therapy and the kidney: what the nephrologist needs to know. Clin J Am Soc Nephrol 13:796–798
Kanduri SR, Cheungpasitporn W, Thongprayoon C, Petnak T, Lin Y, Kovvuru K et al (2021) Systematic review of risk factors and incidence of acute kidney injury among patients treated with CAR-T cell therapies. Kidney Int Rep 6:1416–1422
Myers RM, Fitzgerald J, Elgarten CW, Getz KD, Li Y, Hogan J, Dinofia A, Burrows EK, Aplenc R, Grupp SA, Laskin B, Maude SL (2019) Acute kidney injury after chimeric antigen receptor T-cell therapy for pediatric acute lymphoblastic leukemia. Biol Blood Marrow Transplant 25:S168–S169
Gutgarts V, Jain T, Zheng J, Maloy MA, Ruiz JD, Pennisi M et al (2020) Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood Marrow Transplant 26:1071–1076
Laskin BL, Nehus E, Goebel J, Khoury JC, Davies SM, Jodele S (2012) Cystatin C-estimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 18:1745–1752
Glezerman IG, Devlin S, Maloy M, Bui M, Jaimes EA, Giralt SA et al (2017) Long term renal survival in patients undergoing T-cell depleted versus conventional hematopoietic stem cell transplants. Bone Marrow Transplant 52:733–738
Filler G, Lee M (2018) Educational review: measurement of GFR in special populations. Pediatr Nephrol 33:2037–2046
Talleur AC, Qudeimat A, Métais JY, Langfitt D, Mamcarz E, Crawford JC et al (2022) Preferential expansion of CD8+ CD19-CAR T cells postinfusion and the role of disease burden on outcome in pediatric B-ALL. Blood Adv 6:5737–5749
Benoit SW, Kathman T, Patel J, Stegman M, Cobb C, Hoehn J et al (2021) GFR estimation after cystatin C reference material change. Kidney Int Rep 6:429–436
Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA et al (2012) Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82:445–453
Lameire NH, Levin A, Kellum JA, Cheung M, Jadoul M, Winkelmayer WC et al (2021) Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 100:516–526
Benoit SW, Dixon BP, Goldstein SL, Bennett MR, Lane A, Lounder DT et al (2019) A novel strategy for identifying early acute kidney injury in pediatric hematopoietic stem cell transplantation. Bone Marrow Transplant 54:1453–1461
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL et al (2018) Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 15:47–62
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al (2014) Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG et al (2013) Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122:4129–4139
Pechlaner A, Kropshofer G, Crazzolara R, Hetzer B, Pechlaner R, Cortina G (2022) Mortality of hemato-oncologic patients admitted to a pediatric intensive care unit: a single-center experience. Front Pediatr 10:795158
Fitzgerald JC, Williams D, Laskin BL (2014) Acute kidney injury in pediatric hematopoietic stem cell transplant recipients. J Pediatr Intensive Care 3:159–168
Raina R, Herrera N, Krishnappa V, Sethi SK, Deep A, Kao WM et al (2017) Hematopoietic stem cell transplantation and acute kidney injury in children: A comprehensive review. Pediatr Transplant 21:e12935. https://doi.org/10.1111/petr.12935
Raina R, Abu-Arja R, Sethi S, Dua R, Chakraborty R, Dibb JT et al (2022) Acute kidney injury in pediatric hematopoietic cell transplantation: critical appraisal and consensus. Pediatr Nephrol 37:1179–1203
Hingorani S (2016) Renal complications of hematopoietic-cell transplantation. N Engl J Med 374:2256–2267
Weinstein JR, Anderson S (2010) The aging kidney: physiological changes. Adv Chronic Kidney Dis 17:302–307
Zhou H, Yang M, Cui L, Jiang J (2020) Chimeric antigen receptor T cell therapy and nephrotoxicity: from diagnosis to treatment strategies. Int Immunopharmacol 89(Pt B):107072
Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML et al (2020) Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv 4:676–686
Zappitelli M, Greenberg JH, Coca SG, Krawczeski CD, Li S, Thiessen-Philbrook HR et al (2015) Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr 169:583–591
Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S et al (2010) Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol 5:1745–1754
Nehus EJ, Laskin BL, Kathman TI, Bissler JJ (2013) Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol 28:453–461
Funding
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute grants P30CA021765 and 5P30CA021765-42, the American Society of Hematology (AT), the American Society of Transplantation and Cellular Therapy (AT), and the American Lebanese Syrian Associated Charities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SG consults/consulted for TESSA Therapeutics, TIDAL, Catamaran, and Novartis and is DSMB member of Immatics. SG and RE have patents/patent applications in the fields of T-cell and/or gene therapy for cancer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petgrave, Y., Selukar, S., Epperly, R. et al. Acute kidney injury following treatment with CD19-specific CAR T-cell therapy in children, adolescent, and young adult patients with B-cell acute lymphoblastic leukemia. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06331-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00467-024-06331-7