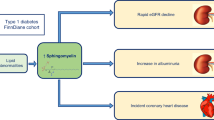
Abstract
Background
This study evaluated urinary sphingolipids as a marker of diabetic kidney disease (DKD) in adolescents and young adults with youth-onset type 1 and type 2 diabetes.
Methods
A comprehensive panel of urinary sphingolipids, including sphingomyelin (SM), glucosylceramide (GC), ceramide (Cer), and lactosylceramide (LC) species, was performed in patients with youth-onset diabetes from the SEARCH for Diabetes in Youth cohort. Sphingolipid levels, normalized to urine creatinine, were compared in 57 adolescents and young adults with type 1 diabetes, 59 with type 2 diabetes, and 44 healthy controls. The association of sphingolipids with albumin-to-creatinine (ACR) ratio and estimated glomerular filtration rate (eGFR) was evaluated.
Results
The median age (interquartile range [IQR]) of participants was 23.1 years (20.9, 24.9) and the median duration of diabetes was 9.3 (8.5, 10.2) years. Urinary sphingolipid concentrations in patients with and without DKD (ACR ≥ 30 mg/g) were significantly elevated compared to healthy controls. There were no significant differences in sphingolipid levels between participants with type 1 and type 2 diabetes. In multivariable analysis, many sphingolipid species were positively correlated with ACR. Most significant associations were evident for the following species: C18 SM, C24:1 SM, C24:1 GC, and C24:1 Cer (all p < 0.001). Sphingolipid levels were not associated with eGFR. However, several interaction terms (diabetes type*sphingolipid) were significant, indicating diabetes type may modify the association of sphingolipids with eGFR.
Conclusion
Urinary sphingolipids are elevated in adolescents and young adults with youth-onset diabetes and correlate with ACR. Urinary sphingolipids may therefore represent an early biomarker of DKD.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information




Similar content being viewed by others
Data availability
Data from the SEARCH for Diabetes in Youth that were used in this study are available for request at the NIDDK Central Repository (NIDDK-CR) website, Resources for Research (R4R), https://repository.niddk.nih.gov/.
References
Saran R, Robinson B, Abbott KC et al (2019) US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 73:A7–A8
Macisaac RJ, Jerums G (2011) Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 20:246–257
Caramori ML, Fioretto P, Mauer M (2003) Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 52:1036–1040
Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis J (2012) Renal dysfunction in the presence of normoalbuminuria in type 2 diabetes: Results from the demand study. Cardiorenal Med 2:1–10
MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G (2004) Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 27:195–200
Lee CH, Lam KS (2015) Biomarkers of progression in diabetic nephropathy: The past, present and future. J Diabetes Investig 6:247–249
Sas KM, Nair V, Byan J et al (2015) Targeted lipidomic and transcriptomic analysis identifies dysregulated renal ceramide metabolism in a mouse model of diabetic kidney disease. J Proteomics Bioinform Suppl 14:002
Liu G, Han F, Yang Y et al (2011) Evaluatin of sphingolipid metabolism in renal cortex of rats with streptoztocin-induced diabetes and the effects of rapamycin. Nephrol Dial Transplant 26:1493–1502
Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA (1993) A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest 91:797–803
Subathra M, Korrapati M, Howell LA et al (2015) Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol 309:F204–F215
Bijl N, Sokolovic M, Vrins C et al (2009) Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology 50:1431–1441
Boon J, Hoy AJ, Stark R et al (2013) Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62:401–410
Griess K, Rieck M, Muller N et al (2023) Sphingolipid subtypes differentially control proinsulin processing and systemic glucose homeostasis. Nat Cell Biol 25:20–29
Aburasayn H, Al Batran R, Ussher JR (2016) Targeting ceramide metabolism in obesity. Am J Physiol Endocrinol Metab 311:E423-435
SEARCH Study Group (2004) SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25:458–471
Hamman RF, Bell RA, Dabelea D et al (2014) The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 37:3336–3344
Pierce CB, Munoz A, Ng DK, Warady BA, Furth SL, Schwartz GJ (2021) Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int 99:948–956
Davis S, Nehus E, Inge T, Zhang W, Setchell K, Mitsnefes M (2018) Effect of bariatric surgery on urinary sphingolipids in adolescents with severe obesity. Surg Obes Relat Dis 14:446–451
Mallela S, Merscher S, Fornoni A (2022) Implications of sphingolipid metabolites in kidnes diseases. Int J Mol Sci 23:4244
Li G, Kidd J, Gehr T, Li P (2021) Podocyte sphingolipid signaling in nephrotic syndrome. Cell Phsiol Biochem 55(Suppl 4):13–34
Roszczyc-Owsiejczuk K, Zabielski P (2021) Sphingolipids as a culprit of mitochondrial dysfunction in insulin resistance and type 2 diabetes. Front Endcrionol 12:635175
Hammand S, Lopes-Virella M (2023) Circulating sphingolipids in insulin resistance, diabetes, and associated complications. Int J Mol Sci 24:14015
Lui J, Ghosh S, Kovalik J et al (2016) Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney Int Rep 2:470–480
Barlovic D, Harjustsalo V, Sandhom N, Forsblom C; FinnDiane Study Group (2020) Sphingomyelin and progression of renal and coronary heart disease in individuals with type 1 diabetes. Diabetologia 63:1847–1856
Makinen V, Tynkkynen T, Soininen P et al (2012) Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics 8:369–375
Morita Y, Kuran M, Sakai E et al (2020) Analysis of urinary sphingolipids usiing liquid chromatography-tandem mass spectrometyr in diabetic nephropathy. J Diabetes Invest 11:441–449
Bikman BT, Summers SA (2011) Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121:4222–4230
Russo SB, Ross JS, Cowart LA (2013) Spingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol 216:373–401
Stathem M, Marimuthu S, O’Neal J et al (2015) Glucose availability and glycolytic metabolism dictate glycosphingolipid levels. J Cell Biochem 116:67–80
Shayman JA (2018) Targeting Glucosylceramide Synthesis in the Treatment of Rare and Common Renal Disease. Semin Nephrol 38:183–192
Tonneijck L, Muskiet MH, Smits MM et al (2017) Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol 28:1023–1039
Lau CE, Siskos AP, Maitre L et al (2018) Determinants of the urinary and serum metabolome in children from six European populations. BMC Med 16:202
Mittelstrass K, Ried JS, Yu Z et al (2011) Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 7:e1002215
Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, Group US (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55:1832–1839
Yu MK, Lyles CR, Bent-Shaw LA, Young BA, Authors P (2012) Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol 36:245–251
Bjornstad P, Cherney DZ (2018) Renal hyperfiltration in adolescents with type 2 diabetes: physiology, sex differences, and implications for diabetic kidney disease. Curr Diab Rep 18:22
Bjornstad P, Nehus E, El Ghormli L et al (2018) Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: An observational Analysis of data from the today clinical trial. Am J Kidney Dis 71:65–74
Lovshin JA, Skrtic M, Bjornstad P et al (2018) Hyperfiltration, urinary albumin excretion, and ambulatory blood pressure in adolescents with Type 1 diabetes mellitus. Am J Physiol Renal Physiol 314:F667–F674
Silverstein J, Klingensmith G, Copeland K et al (2005) Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 28:186–212
Tagami S, Inokuchi Ji J, Kabayama K et al (2002) Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem 277:3085–3092
Yamashita T, Hashiramoto A, Haluzik M et al (2003) Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A 100:3445–3449
Acknowledgements
Personal thanks The SEARCH for Diabetes in Youth Study is indebted to the many youths and their families, and their health care providers, whose participation made this study possible.
SEARCH 3/ 4
The authors wish to acknowledge the involvement of the Kaiser Permanente Southern California’s Marilyn Owsley Clinical Research Center (funded by Kaiser Foundation Health Plan and supported in part by the Southern California Permanente Medical Group); the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina; NIH/National Center for Advancing Translational Sciences (NCATS) grant number UL1 TR000062 and UL1 Tr001450; Seattle Children's Hospital and the University of Washington, NIH/NCATS grant number UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant Number UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (DERC NIH grant number P30 DK57516); the University of Cincinnati, NIH/NCATS grant number UL1 TR000077 and UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Contributions
EN wrote the manuscript and performed the analysis. NS, SM, and MM contributed to the discussion and reviewed/edited the manuscript. KDRS and WZ developed and validated the urinary sphingolipid analyses and performed laboratory analyses and data interpretation. EN is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior presentation This data was presented in abstract form at the American Society of Nephrology meeting, 2020 (virtual only).
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nehus, E.J., Sheanon, N.M., Zhang, W. et al. Urinary sphingolipids in adolescents and young adults with youth-onset diabetes. Pediatr Nephrol 39, 1875–1883 (2024). https://doi.org/10.1007/s00467-023-06257-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06257-6