Abstract
Background
Neuroendocrine tumors (NETs) are a group of heterogenous tumors originating from neuroendocrine system. Approximately, 40 percent will go through liver metastases, and liver-directed therapy was proved to improve the survival outcome. Parenchyma-sparing hepatectomy is advocated for the resection of NETs liver metastases while the possible relatively low negative margin rate is concerned. Indocyanine green (ICG) fluorescence imaging provides a real-time navigation on determination of surgical margins in colorectal cancer liver metastases. However, there was no previous study that reported the applications of ICG fluorescence imaging in NETs liver metastases. The present study aimed to evaluate the feasibility and security of using ICG fluorescence imaging to determine surgical margins of NETs liver metastases during operation.
Methods
A retrospective two-arm cohort study was performed on 25 consecutive patients with NETs liver metastases who underwent laparoscopic parenchyma-sparing hepatectomy (LPSH). Patients were divided into two groups according to whether or not the ICG fluorescence imaging was used. Data on sociodemographic characteristics, laboratory parameters, pathology results, and surgical outcomes were collected.
Results
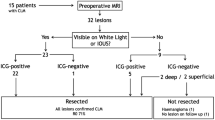
A total of 145 tumors pathologically diagnosed with NETs liver metastases were resected from 25 patients. The pathological results indicated negative margins in all tumors (102/102) in LPSH with ICG fluorescence imaging group. The negative margin rate was significantly higher in LPSH using the ICG fluorescence imaging (100% v.s 88.4%, p = 0.002). Surgical outcomes, including operation time, estimated blood loss, intraoperative transfusion rate, and postoperative morbidity, were comparable between LPSH with and without ICG fluorescence imaging groups.
Conclusion
ICG fluorescence imaging showed the potential to identify tumor boundaries and determine surgical margins. This technique may serve as a valuable intraoperative navigation in patients with NETs liver metastases.



Similar content being viewed by others
References
Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S (2015) Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121:589–597
Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A (2015) Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 50:58–64
Pape UF, Bohmig M, Berndt U, Tiling N, Wiedenmann B, Plockinger U (2004) Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann N Y Acad Sci 1014:222–233
Frilling A, Modlin IM, Kidd M, Russell C, Breitenstein S, Salem R, Kwekkeboom D, Lau WY, Klersy C, Vilgrain V, Davidson B, Siegler M, Caplin M, Solcia E, Schilsky R, M. Working Group on Neuroendocrine Liver (2014) Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 15:e8-21
Mayo SC, de Jong MC, Pulitano C, Clary BM, Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Ferrero A, Schulick RD, Choti MA, Mentha G, Strub J, Bauer TW, Adams RB, Aldrighetti L, Capussotti L, Pawlik TM (2010) Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 17:3129–3136
Maxwell JE, Sherman SK, O’Dorisio TM, Bellizzi AM, Howe JR (2016) Liver-directed surgery of neuroendocrine metastases: What is the optimal strategy? Surgery 159:320–333
Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL (2002) Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235:759–766
Liu B, Liu T, Su M, Ma YQ, Zhang BF, Wang YF, Hu BY, Chen YL (2019) Improving the surgical effect for primary liver cancer with intraoperative fluorescence navigation compared with intraoperative ultrasound. Med Sci Monit 25:3406–3416
Barabino G, Porcheron J, Cottier M, Cuilleron M, Coutard JG, Berger M, Molliex S, Beauchesne B, Phelip JM, Grichine A, Coll JL (2016) Improving surgical resection of metastatic liver tumors with near-infrared optical-guided fluorescence imaging. Surg Innov 23:354–359
Majlesara A, Golriz M, Hafezi M, Saffari A, Stenau E, Maier-Hein L, Muller-Stich BP, Mehrabi A (2017) Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn Ther 17:208–215
Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, Hasegawa K, Beck Y, Fukayama M, Kokudo N (2009) Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 115:2491–2504
Aoki T, Murakami M, Koizumi T, Matsuda K, Fujimori A, Kusano T, Enami Y, Goto S, Watanabe M, Otsuka K (2018) Determination of the surgical margin in laparoscopic liver resections using infrared indocyanine green fluorescence. Langenbecks Arch Surg 403:671–680
Zhou Y, Lin Y, Jin H, Hou B, Yu M, Yin Z, Jian Z (2019) Real-time navigation guidance using fusion indocyanine green fluorescence imaging in laparoscopic non-anatomical hepatectomy of hepatocellular carcinomas at segments 6, 7, or 8 (with videos). Med Sci Monit 25:1512–1517
Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, Ma L, Qi LN, Ou BN, Li LQ (2016) Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 103:725–734
Pereyra D, Rumpf B, Ammann M, Perrodin SF, Tamandl D, Haselmann C, Stift J, Brostjan C, Laengle F, Beldi G, Gruenberger T, Starlinger P (2019) The Combination of APRI and ALBI facilitates preoperative risk stratification for patients undergoing liver surgery after neoadjuvant chemotherapy. Ann Surg Oncol 26:791–799
Torzilli G, McCormack L, Pawlik T (2020) Parenchyma-sparing liver resections. Int J Surg 82S:192–197
Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, Antoniou A (2007) Laparoscopic versus open hepatic resections for benign and malignant neoplasms–a meta-analysis. Surgery 141:203–211
Lai IR, Yeh CC, Yu SC (2009) Laparoscopic liver resection for hepatocellular carcinoma: intermediate follow-up results. Hepatogastroenterology 56:1082–1085
Meguro M, Mizuguchi T, Kawamoto M, Ota S, Ishii M, Nishidate T, Okita K, Kimura Y, Hirata K (2015) Clinical comparison of laparoscopic and open liver resection after propensity matching selection. Surgery 158:573–587
Altendorf-Hofmann A, Scheele J (2003) A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 12:165–192
Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghemard O, Levi F, Bismuth H (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240:644–657
Elias D, Lasser P, Ducreux M, Duvillard P, Ouellet JF, Dromain C, Schlumberger M, Pocard M, Boige V, Miquel C, Baudin E (2003) Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery 133:375–382
Cho CS, Labow DM, Tang L, Klimstra DS, Loeffler AG, Leverson GE, Fong Y, Jarnagin WR, D’Angelica MI, Weber SM, Blumgart LH, Dematteo RP (2008) Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer 113:126–134
Saxena A, Chua TC, Sarkar A, Chu F, Liauw W, Zhao J, Morris DL (2011) Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery 149:209–220
Cherrick GR, Stein SW, Leevy CM, Davidson CS (1960) Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest 39:592–600
Tashiro Y, Aoki T, Hirai T, Koizumi T, Mansou DA, Kusano T, Matsuda K, Yamada K, Nogaki K, Hakozaki T, Wada Y, Shibata H, Tomioka K, Yamazaki T, Saito K, Fujimori A, Enami Y, Hoffman RM, Murakami M (2020) Pathological validity of using near-infrared fluorescence imaging for securing surgical margins during liver resection. Anticancer Res 40:3873–3882
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
All authors had the idea for and designed the study. GW, YL, CY, WQ, and DX contributed to the literature search. GW, YL, WQ, and CY contributed to data collection. GW, YL, CY, and DX contributed to data analysis and interpretations. GW and YL contributed to the figures and tables. GW and YL contributed to writing of the manuscript. All authors revised the final manuscript. All authors approved the final version of the article, including the authorship list.
Corresponding author
Ethics declarations
Disclosures
Gaoming Wang, Ying Luo, Weijun Qi, Chunhui Yuan, and Dianrong Xiu have no conflicts of interest or financial ties to disclose.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. The study was approved by the institutional review board of Peking University Third Hospital (M2021257).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, G., Luo, Y., Qi, W. et al. Determination of surgical margins in laparoscopic parenchyma-sparing hepatectomy of neuroendocrine tumors liver metastases using indocyanine green fluorescence imaging. Surg Endosc 36, 4408–4416 (2022). https://doi.org/10.1007/s00464-021-08791-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08791-6