Abstract
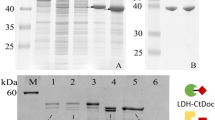
Chiral compounds are important drug intermediates that play a critical role in human life. Herein, we report a facile method to prepare multi-enzyme nano-devices with high catalytic activity and stability. The self-assemble molecular binders SpyCatcher and SpyTag were fused with leucine dehydrogenase and glucose dehydrogenase to produce sc-LeuDH (SpyCatcher-fused leucine dehydrogenase) and GDH-st (SpyTag-fused glucose dehydrogenase), respectively. After assembling, the cross-linked enzymes LeuDH-GDH were formed. The crosslinking enzyme has good pH stability and temperature stability. The coenzyme cycle constant of LeuDH-GDH was always higher than that of free double enzymes. The yield of l-tert-leucine synthesis by LeuDH-GDH was 0.47 times higher than that by free LeuDH and GDH. To further improve the enzyme performance, the cross-linked LeuDH-GDH was immobilized on zeolite imidazolate framework-8 (ZIF-8) via bionic mineralization, forming LeuDH-GDH @ZIF-8. The created co-immobilized enzymes showed even better pH stability and temperature stability than the cross-linked enzymes, and LeuDH-GDH@ZIF-8 retains 70% relative conversion rate in the first four reuses. In addition, the yield of LeuDH-GDH@ZIF-8 was 0.62 times higher than that of LeuDH-GDH, and 1.38 times higher than that of free double enzyme system. This work provides a novel method for developing multi-enzyme nano-device, and the ease of operation of this method is appealing for the construction of other multi-enzymes @MOF systems for the applications in the kinds of complex environment.
Graphical abstract









Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
03 August 2023
The original online version of this article was revised: To update the given and the family name of the authors
02 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00449-023-02916-y
References
Gladiali S (2007) Guidelines and methodologies in asymmetric synthesis and catalysis. C R Chim 10(3):220–231
Luo W et al (2020) Cloning and expression of a novel leucine dehydrogenase: characterization and l-tert-Leucine production. Front Bioeng Biotechnol 8:186
Jiang W, Fang B (2020) Synthesizing chiral drug intermediates by biocatalysis. Appl Biochem Biotechnol 192(1):146–179
Solano DM et al (2012) Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Biores Technol 115:196–207
Menzel A et al (2004) From enzymes to “designer bugs” in reductive amination: a new process for the synthesis of L-tert-leucine using a whole cell-catalyst. Eng Life Sci 4(6):573–576
Bommarius AS, Schwarm M, Drauz K (1998) Biocatalysis to amino acid-based chiral pharmaceuticals—examples and perspectives. J Mol Catal B Enzym 5(1–4):1–11
Young Hong E et al (2010) Asymmetric synthesis of L-tert-leucine and L-3-hydroxyadamantylglycine using branched chain aminotransferase. J Mol Catal B Enzym 66(1–2):228–233
Jin J-Z, Chang D-L, Zhang J (2011) Discovery and application of new bacterial strains for asymmetric synthesis of l-tert-butyl leucine in high enantioselectivity. Appl Biochem Biotechnol 164(3):376–385
Liu W et al (2014) Efficient synthesis of l-tert-leucine through reductive amination using leucine dehydrogenase and formate dehydrogenase coexpressed in recombinant E. coli. Biochem Eng J 91:204–209
Clive DL, Etkin N (1994) Synthesis of α-amino acids by addition of putative azido radicals to α-methoxy acrylonitriles derived from aldehydes and ketones. Tetrahedron Lett 35(16):2459–2462
Boesten WH et al (2001) Asymmetric Strecker synthesis of α-amino acids via a crystallization-induced asymmetric transformation using (R)-phenylglycine amide as chiral auxiliary. Org Lett 3(8):1121–1124
Drauz K, Gröger H, May O (2012) Enzyme catalysis in organic synthesis, 3 volume set, vol 1. John Wiley & Sons, New Jersey
Yang X et al (2016) High efficient co-expression of leucine dehydrogenase and glucose dehydrogenase in Escherichia coli. Acta Microbiol Sin 56(11):1709–1718
Shu-ting LI, Min W (2009) Research on conditions of l-tert-leucine biosynthesis by genetically engineered Escherichia coli. Pharm Biotechnol 16(3):202–206
Bülow L, Ljungcrantz P, Mosbach K (1985) Preparation of a soluble bifunctional enzyme by gene fusion. Bio/Technology 3(9):821–823
Fan Z et al (2009) Multimeric hemicellulases facilitate biomass conversion. Appl Environ Microbiol 75(6):1754–1757
Yerabham AS et al (2013) Revisiting disrupted-in-schizophrenia 1 as a scaffold protein. Biol Chem 394(11):1425–1437
Hyeon JE, Jeon SD, Han SO (2013) Cellulosome-based, Clostridium-derived multi-functional enzyme complexes for advanced biotechnology tool development: advances and applications. Biotechnol Adv 31(6):936–944
Zakeri B et al (2012) Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci 109(12):E690–E697
Hagan RM et al (2010) NMR spectroscopic and theoretical analysis of a spontaneously formed Lys-Asp isopeptide bond. Angew Chem 122(45):8599–8603
Banerjee A, Howarth M (2018) Nanoteamwork: covalent protein assembly beyond duets towards protein ensembles and orchestras. Curr Opin Biotechnol 51:16–23
Reddington SC, Howarth M (2015) Secrets of a covalent interaction for biomaterials and biotechnology: SpyTag and SpyCatcher. Curr Opin Chem Biol 29:94–99
Liu D et al (2017) Topology engineering of proteins in vivo using genetically encoded, mechanically interlocking SpyX modules for enhanced stability. ACS Cent Sci 3(5):473–481
Liu L et al (2018) Construction of intracellular self-assembled multienzyme complex by SpyTag/SpyCatcher to achieve efficient biosynthesis. China Biotechnol 38(7):75–82
Dordick JS (1992) Designing enzymes for use in organic solvents. Biotechnol Prog 8(4):259–267
Cantone S et al (2013) Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem Soc Rev 42(15):6262–6276
Bailey JB et al (2017) Synthetic modularity of protein–metal–organic frameworks. J Am Chem Soc 139(24):8160–8166
Pilgrim BS, Champness NR (2020) Metal-organic frameworks and metal-organic cages–a perspective. Chem Plus Chem 85(8):1842–1856
Bai W et al (2019) Ultrathin 2D metal–organic framework (nanosheets and nanofilms)-based x D–2D hybrid nanostructures as biomimetic enzymes and supercapacitors. J Mater Chem A 7(15):9086–9098
Chen GS et al (2020) Embedding functional biomacromolecules within peptide-directed metal-organic framework (MOF) nanoarchitectures enables activity enhancement. Angew Chem Int Edn 59(33):13947–13954
Liang W et al (2021) Metal–organic framework-based enzyme biocomposites. Chem Rev 121(3):1077–1129
Liang K et al (2017) Biomimetic mineralization of metal–organic frameworks around polysaccharides. Chem Commun 53(7):1249–1252
Gao X et al (2021) Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: a kinetic and thermodynamic study of structure-activity. Catal Sci Technol 11(7):2446–2455
Liao F-S et al (2017) Shielding against unfolding by embedding enzymes in metal–organic frameworks via a de novo approach. J Am Chem Soc 139(19):6530–6533
Qi B, Luo J, Wan Y (2018) Immobilization of cellulase on a core-shell structured metal-organic framework composites: better inhibitors tolerance and easier recycling. Biores Technol 268:577–582
Liang K et al (2015) Biomimetic mineralization of metal–organic frameworks as protective coatings for biomacromolecules. Nat Commun 6(1):1–8
Akimbekov Z et al (2017) Experimental and theoretical evaluation of the stability of true MOF polymorphs explains their mechanochemical interconversions. J Am Chem Soc 139(23):7952–7957
Acknowledgements
The researchers are grateful for the support given by the National Natural Science Foundation of China (21878154) and the China Postdoctoral Science Foundation (2021M691624).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Jia, J., Liu, X. et al. Construction of metal–organic framework-based multienzyme system for l-tert-leucine production. Bioprocess Biosyst Eng 46, 1365–1373 (2023). https://doi.org/10.1007/s00449-023-02900-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02900-6