Abstract
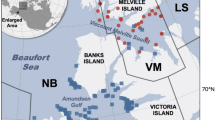
Polar bears (Ursus maritimus) rely on annual sea ice as their primary habitat for hunting marine mammal prey. Given their long lifespan, wide geographic distribution, and position at the top of the Arctic marine food web, the diet composition of polar bears can provide insights into temporal and spatial ecosystem dynamics related to climate-mediated sea ice loss. Polar bears with the greatest ecological constraints on diet composition may be most vulnerable to climate-related changes in ice conditions and prey availability. We used quantitative fatty acid signature analysis (QFASA) to estimate the diets of polar bears (n = 419) in two western Canadian Arctic subpopulations (Northern Beaufort Sea and Southern Beaufort Sea) from 1999 to 2015. Polar bear diets were dominated by ringed seal (Pusa hispida), with interannual, seasonal, age- and sex-specific variation. Foraging area and sea ice conditions also affected polar bear diet composition. Most variation in bear diet was explained by longitude, reflecting spatial variation in prey availability. Sea ice conditions (extent, thickness, and seasonal duration) declined throughout the study period, and date of sea ice break-up in the preceding spring was positively correlated with female body condition and consumption of beluga whale (Delphinapterus leucas), suggesting that bears foraged on beluga whales during entrapment events. Female body condition was positively correlated with ringed seal consumption, and negatively correlated with bearded seal consumption. This study provides insights into the complex relationships between declining sea ice habitat and the diet composition and foraging success of a wide-ranging apex predator.







Similar content being viewed by others
References
Ackman RG, Eaton CA (1966) Some commercial Atlantic herring oils; fatty acid composition. Fish Res Bd Can 23:991–1006
Ashjian CJ et al (2010) Climate variability, oceanography, bowhead whale distribution, and Iñupiat subsistence whaling near Barrow Alaska. Arctic 63:179–194
Bentzen T et al (2007) Variation in winter diet of southern Beaufort Sea polar bears inferred from stable isotope analysis. Can J Zool 85:596–608
Borcard D et al (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Boucher N et al (2019) Variability in polar bear Ursus maritimus stable isotopes in relation to environmental change in the Canadian Beaufort Sea. Mar Ecol Prog Ser 630:215–225
Bowen WD (1997) Role of marine mammals in aquatic ecosystems. Mar Ecol Prog Ser 158:267–274
Bromaghin JF et al (2015) Polar bear population dynamics in the Southern Beaufort Sea during a period of sea ice decline. Ecol Appl 25:634–651
Bromaghin JF (2017) qfasar: quantitative fatty acid signature analysis with R. Methods Ecol Evol 8:1158–1162
Budge SM et al (2006) Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar Mammal Sci 22:759–801
Calvert W, Ramsay MA (1998) Evaluation of age determination of polar bears by counts of cementum growth layer groups. Ursus 10:449–453
Calvert W, Stirling I (1989) Interactions between polar bears and overwintering walruses in the central Canadian High Arctic. In: Their biology and management, Eight International Conference on Bear Research and Management, Victoria, British Columbia, Canada. International Association for Bear Research and Management, pp. 351–356
Chambellant M et al (2012) Temporal variations in Hudson Bay ringed seal (Phoca hispida) life-history parameters in relation to environment. J Mammal 93:267–281
Cherry SG et al (2010) Quantifying dietary pathways of proteins and lipids to tissues of a marine predator. J Appl Ecol 48:373–381
Derocher AE et al (2002) Diet composition of polar bears in Svalbard and the western Barents Sea. Polar Biol 25:448–452
Derocher AE et al (2005) Sexual dimorphism of polar bears. -. J Mammal 86:895–901
Derocher AE et al (2010) Sexual dimorphism and the mating ecology of polar bears (Ursus maritimus) at Svalbard. Behav Ecol Sociobiol 64:939–946
Durner GM et al (2017) Increased Arctic sea ice drift alters adult female polar bear movements and energetics. Glob Change Biol 23:3460–3473
Durner GM, Laidre KL, York, GS (eds) (2018) Polar Bears: Proceedings of the 18th Working Meeting of the IUCN/SSC Polar Bear Specialist Group, 7–11 June 2016, Anchorage, Alaska. Gland, Switzerland and Cambridge, UK, IUCN, pp. 287
Ferguson SH et al (2005) Climate change and ringed seal (Phoca hispida) recruitment in Western Hudson Bay. Mar Mammal Sci 21:121–135
Ferguson SH et al (2017) Demographic, ecological and physiological responses of ringed seals to an abrupt decline in sea ice availability. PeerJ 5:e2957. https://doi.org/10.7717/peerj.2957
Ferguson SH, Higdon JW (2006) How seals divide up the world: environment, life history, and conservation. Oecologia 150:318–329
Folch J et al (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Freeman MMF (1973) Polar bear predation on beluga in the Canadian Arctic. Arctic 26:162–163
Galicia MP et al (2015) Characterization of polar bear (Ursus maritimus) diets in the Canadian high arctic. Polar Biol 38:1983–1992
Galicia MP et al (2016) Dietary habits of polar bears in Foxe Basin, Canada: possible evidence of a trophic regime shift mediated by a new top predator. Ecol Evol 6:6005–6018
Hamilton CD et al (2017) An Arctic predator–prey system in flux: climate change impacts on coastal space use by polar bears and ringed seals. J Anim Ecol 38:42–49
Harwood LA et al (2000) Variation in reproduction and body condition of the ringed seal (Phoca hispida) in western Prince Albert Sound, NT, Canada, as assessed through a harvest-based sampling program. Arctic 53:422–431
Harwood LA et al (2012) Ringed seals and sea ice in Canada’s western Arctic: harvest-based monitoring 1992–2011. Arctic 65:377–390
Harwood LA et al (2014) An emerging pattern of declining growth rates in belugas of the Beaufort Sea: 1989–2008. Arctic 67:483–492
Harwood LA et al (2015) Seasonal movements and diving of ringed seals, Pusa hispida, in the Western Canadian Arctic, 1999–2001 and 2010–11. Arctic 68:193–209
Harwood LA, Kingsley MCS (2013) Trends in the offshore distribution and relative abundance of Beaufort Sea belugas, 1982–85 vs 2007–09. Arctic 66:247–256
Harwood LA, Smith TG (2002) Whales of the Inuvialuit settlement region in Canada’s Western Arctic: an overview and outlook. Arctic 55:77–93
Herreman J, Peacock E (2013) Polar bear use of a persistent food subsidy: insights from non-invasive genetic sampling in Alaska. Ursus 24:148–163
Higdon JW, Ferguson SH (2012) Environmental conditions and beluga whale entrapment events in the Husky Lakes, NWT. Canada/Inuvialuit Fisheries Joint Management Committee Report 2012. Canada/Inuvialuit Fisheries Joint Management Committee Report 2012
Hornby C et al (2014) Arrival of Beluga (Delphinapterus leucas) to the Mackenzie estuary in relation to sea ice: report on spring 2011–2013 aerial surveys. Freshwater Institute, Fisheries and Oceans Canada
Hornby CA et al (2016) Spring conditions and habitat use of beluga whales (Delphinapterus leucas) during arrival to the Mackenzie River Estuary. Polar Biol 39:2319–2334
Hornby CA et al (2017) Beluga whale Delphinapterus leucas late summer habitat use and support for foraging areas in the Canadian Beaufort Sea. Mar Ecol Prog Ser 574:243–257
Horswill C et al (2016) Unravelling the relative roles of top-down and bottom-up forces driving population change in an oceanic predator. Ecology 97:1919–1928
Huntington HP et al (1999) Traditional Knowledge of the ecology of beluga whales (Delphinapterus lencas) in the Eastern Chukchi and Northern Bering Seas, Alaska. Arctic 52:49–61
Huntington HP (2002) Traditional knowledge of the ecology of belugas, Delphinapterus leucas, in Cook Inlet Alaska. Mar Fish Rev 62:134–140
Iverson SJ et al (2004) Quantitative fatty acid signature analysis: a new method of estimating predator diets. Ecol Monogr 74:211–235
Katona S, Whitehead H (1988) Are Cetacea ecologically important? Oceanogr Mar Biol Annu Rev 26:553–568
Kingsley MCS et al (1985) The distribution and abundance of seals in the Canadian high Arctic. Can J Fish Aquat Sci 42:1189–1210
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Lillie K et al (2019) Use of subsistence-harvested whale carcasses by polar bears in the Southern Beaufort Sea. Arctic 72:404–412
Lindsay R, Schweiger A (2015) Arctic sea ice thickness loss determined using subsurface, aircraft, and satellite observations. Cryosph 9:269–283
Lone K et al (2018) Sea ice resource selection models for polar bears in the Barents Sea subpopulation. Ecography 41:567–578
Lunn NJ et al (2016) Demography of an apex predator at the edge of its range—impacts of changing sea ice on polar bears in Hudson Bay. Ecol Appl 26:1302–1320
Luque SP, Ferguson SH (2009) Ecosystem regime shifts have not affected growth and survivorship of eastern Beaufort Sea belugas. Oecologia 160:367–378
Maslanik J et al (2011) Distribution and trends in Arctic sea ice age through spring 2011. Geophys Res Lett 38:2–7
McKinney MA et al (2014) Validation of adipose lipid content as a body condition index for polar bears. Ecol Evol 4:516–527
McKinney MA et al (2017) Temporal complexity of southern Beaufort Sea polar bear diets during a period of increasing land use. Ecosphere 8:e01633. https://doi.org/10.1002/ecs2.1633
Obbard ME et al (2016) Trends in body condition in polar bears (Ursus maritimus) from the Southern Hudson Bay subpopulation in relation to changes in sea ice. Arct Sci 2:15–32
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Peacock E et al (2012) The utility of harvest recoveries of marked individuals to assess polar bear (Ursus maritimus) survival. Arctic 65:391–400
Pickart RS et al (2013) Long-term trends of upwelling and impacts on primary productivity in the Alaskan Beaufort Sea. Deep Sea Res Part I Oceanogr Res Pap 79:106–121
Pilfold NW et al (2014) Polar bear predatory behaviour reveals seascape distribution of ringed seal lairs. Popul Ecol 56:129–138
Pilfold NW et al (2015) Multi-temporal factors influence predation for polar bears in a changing climate. Oikos 124:1098–1107
Pond CM (1992) An evolutionary and functional view of mammalian adipose tissue. Proc Nutr Soc 51:367–377
Quakenbush L et al (2011) Biology of the bearded seal (Erignathus barbatus) in Alaska, 1961–2009. Final Report to National Marine Fisheries Service
Regehr EV et al (2007) Effects of earlier sea ice breakup on survival and population size of polar bears in Western Hudson Bay. J Wildl Manage 71:2673–2683
Regehr EV et al (2016) Conservation status of polar bears (Ursus maritimus) in relation to projected sea-ice declines. Biol Lett 12:20160556
Reimer JR et al (2019) Ringed seal demography in a changing climate. Ecol Appl 29(3):e01855
Rode KD et al (2010) Reduced body size and cub recruitment in polar bears associated with sea ice decline. Ecol Appl 20:768–782
Rode KD et al (2014) Variation in the response of an Arctic top predator experiencing habitat loss: feeding and reproductive ecology of two polar bear populations. Glob Change Biol 20:76–88
Schliebe S et al (2008) Effects of sea ice extent and food availability on spatial and temporal distribution of polar bears during the fall open-water period in the Southern Beaufort Sea. Polar Biol 31:999–1010
Schulze LM, Pickart RS (2012) Seasonal variation of upwelling in the Alaskan Beaufort Sea: impact of sea ice cover. J Geophys Res Ocean. https://doi.org/10.1029/2012JC007985
Sciullo L et al (2016) Comparative assessment of metrics for monitoring the body condition of polar bears in western Hudson Bay. J Zool 300:45–58
Sciullo L, Thiemann GW, Lunn NJ, Ferguson SH (2017) Intraspecific and temporal variability in the diet composition of female polar bears in a seasonal sea ice regime. Arct Sci 3(4):672–688
Smith TG (1980) Polar bear predation of ringed and bearded seals in the land-fast sea ice habitat. Can J Zool 58:2201–2209
Smith TG, Sjare B (1990) Predation of belugas and narwhals by polar bears in nearshore areas of the Canadian High Arctic. Arctic 43:99–102
Smith TG, Stirling I (1975) The breeding habitat of the ringed seal (Phoca hispida). The birth lair and associated structures. Can J Zool 53:1297–1305
Stern HL, Laidre KL (2016) Sea-ice indicators of polar bear habitat. Cryosph 10:2027–2041
Stirling I et al (1999) Long-term trends in the population ecology of polar bears in western Hudson Bay in relation to climatic change. Arctic 52:294–306
Stirling I (2002) Polar bears and seals in the eastern Beaufort Sea and Amundsen Gulf: a synthesis of population trends and ecological relationships over three decades. Arctic 55:59–76
Stirling I et al (2011) Polar bear population status in the northern Beaufort Sea, Canada, 1971–2006. Ecol Appl 21:859–876
Stirling I, Derocher AE (1993) Possible impacts of climatic warming on polar bears. Arctic 46:240–245
Stirling I, Oritsland NA (1995) Relationships between estimates of ringed seal (Phoca hispida) and polar bear (Ursus maritimus) populations in the Canadian Arctic. Can J Fish Aquat Sci 52:2594–2612
Stroeve JC et al (2012) Trends in Arctic sea ice extent from CMIP5, CMIP3 and observations. Geophys Res Lett 39:1–7
Stroeve J, Notz D (2015) Insights on past and future sea-ice evolution from combining observations and models. Glob Planet Change 135:119–132
Thiemann GW et al (2004) Determining blubber fatty acid composition: a comparison of in situ direct and traditional methods. Mar Mammal Sci 20:284–295
Thiemann GW et al (2006) Seasonal, sexual and anatomical variability in the adipose tissue of polar bears (Ursus maritimus). J Zool 269:65–76
Thiemann GW et al (2007) Unusual fatty acid biomarkers reveal age- and sex-specific foraging in polar bears (Ursus maritimus). Can J Zool 85:505–517
Thiemann GW et al (2008a) Polar bear diets and Arctic marine food webs: insights from fatty acid analysis. Ecol Monogr 78:591–613
Thiemann GW et al (2008b) Variation in blubber fatty acid composition among marine mammals in the Canadian Arctic. Mar Mammal Sci 24:91–111
Togunov RR et al (2017) Windscapes and olfactory foraging in a large carnivore. Sci Rep 7:46332
van den Wollenberg AL (1977) Redundancy analysis an alternative for canonical correlation analysis. Psychometrika 42:207–219
Wang M, Overland JE (2015) Projected future duration of the sea-ice-free season in the Alaskan Arctic. Prog Oceanogr 136:50–59
Wassmann P et al (2011) Footprints of climate change in the Arctic marine ecosystem. Glob Chang Biol 17:1235–1249
Wood KR et al (2013) Is there a “new normal” climate in the Beaufort Sea? Polar Res 32:19552
Young BG et al (2015) Variation in ringed seal density and abundance in western Hudson Bay estimated from aerial surveys, 1995 to 2013. Arctic 68:301–309
Yurkowski D et al (2016) Influence of sea ice phenology on the movement ecology of ringed seals across their latitudinal range. Mar Ecol Prog Ser 562:237–250
Acknowledgements
We are particularly grateful to the Inuvialuit Game Council and the Inuvialuit hunters for collecting adipose tissue samples from polar bears harvested during their annual subsistence hunts. We thank Marsha Branigan and Jodie Pongracz (Government of Northwest Territories) for organizing and facilitating sample collection and providing ecological insights on the Beaufort Sea ecosystem. This work was supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to GWT, the Kenneth M. Molson Foundation, Northern Scientific Training Program, and York University, Faculty of Graduate Studies. We thank S. Budge and C. Barry (Dalhousie University) for conducting gas chromatography, L. Harwood (Fisheries and Oceans Canada) and J. Alikamik for ringed seal adipose tissue samples, and H. Stern and K. Laidre for access to sea ice data. We thank K. Rode for helpful comments on an earlier draft of this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not constitute endorsement by the U.S. Government. This article is a U.S. Government work and is in the public domain in the U.S.A. This article was reviewed and approved under the U.S. Geological Survey, Fundamental Science Practices policy (https://www.usgs.gov/fsp).
Author information
Authors and Affiliations
Contributions
KRNF and GWT conceived and formulated the ideas, KRNF processed tissue samples, KRNF and JFB analysed the data, KRNF and GWT wrote the manuscript, and JFB provided editorial advice and ecological insights.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of animal rights
The polar bear subsistence hunt and sample collection were carried out under Canadian law.
Additional information
Communicated by Hannu Ylonen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Florko, K.R.N., Thiemann, G.W. & Bromaghin, J.F. Drivers and consequences of apex predator diet composition in the Canadian Beaufort Sea. Oecologia 194, 51–63 (2020). https://doi.org/10.1007/s00442-020-04747-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-020-04747-0