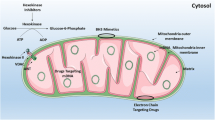
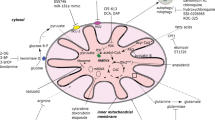
Abstract
Multiple myeloma (MM) is the second most common hematological cancer that has no cure. Although currently there are several novel drugs, most MM patients experience drug resistance and disease relapse. The results of previous studies suggest that aberrant mitochondrial function may contribute to tumor progression and drug resistance. Mitochondrial DNA mutations and metabolic reprogramming have been reported in MM patients. Several preclinical and clinical studies have shown encouraging results of mitochondria-targeting therapy in MM patients. In this review, we have summarized our current understanding of mitochondrial biology in MM. More importantly, we have reviewed mitochondrial targeting strategies in MM treatment.
Similar content being viewed by others
Data availability
Not applicable.
Change history
23 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00432-023-04843-7
References
Allen JE, Krigsfeld G, Mayes PA et al (2013) Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med 5(171):117r–171r
Allen JE, Krigsfeld G, Patel L et al (2015) Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol Cancer 14(1):99
Annunziata CM, Davis RE, Demchenko Y et al (2007) Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12(2):115–130
Bajpai R, Sharma A, Achreja A et al (2020) Electron transport chain activity is a predictor and target for venetoclax sensitivity in multiple myeloma. Nat Commun 11(1):1228
Bellafante E, Morgano A, Salvatore L et al (2014) PGC-1beta promotes enterocyte lifespan and tumorigenesis in the intestine. Proc Natl Acad Sci USA 111(42):E4523–E4531
Bengsch B, Johnson AL, Kurachi M et al (2016) Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 45(2):358–373
Besse L, Besse A, Mendez-Lopez M et al (2019) A metabolic switch in proteasome inhibitor-resistant multiple myeloma ensures higher mitochondrial metabolism, protein folding and sphingomyelin synthesis. Haematologica 104(9):e415–e419
Bhalla K, Hwang BJ, Dewi RE et al (2011) PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res 71(21):6888–6898
Bisping G, Leo R, Kienast J et al (2003) Paracrine interactions of basic fibroblast growth factor and interleukin-6 in multiple myeloma. Blood 101(7):2775–2783
Bodet L, Menoret E, Descamps G et al (2010) BH3-only protein Bik is involved in both apoptosis induction and sensitivity to oxidative stress in multiple myeloma. Br J Cancer 103(12):1808–1814
Bodet L, Gomez-Bougie P, Touzeau C et al (2011) ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood 118(14):3901–3910
Bolzoni M, Chiu M, Accardi F et al (2016) Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: a new attractive target. Blood 128(5):667–679
Bustany S, Bourgeais J, Tchakarska G et al (2016) Cyclin D1 unbalances the redox status controlling cell adhesion, migration, and drug resistance in myeloma cells. Oncotarget 7(29):45214–45224
Bustoros M, Sklavenitis-Pistofidis R, Park J et al (2020) Genomic profiling of smoldering multiple myeloma identifies patients at a high risk of disease progression. J Clin Oncol 38(21):2380–2389
Carracedo A, Cantley LC, Pandolfi PP (2013) Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer 13(4):227–232
Chamoto K, Chowdhury PS, Kumar A et al (2017) Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci USA 114(5):E761–E770
Chauhan D, Neri P, Velankar M et al (2007) Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood 109(3):1220–1227
Chesi M, Mirza NN, Garbitt VM et al (2016) IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat Med 22(12):1411–1420
Chng WJ, Huang GF, Chung TH et al (2011) Clinical and biological implications of MYC activation: a common difference between MGUS and newly diagnosed multiple myeloma. Leukemia 25(6):1026–1035
Cowan AJ, Green DJ, Kwok M et al (2022) Diagnosis and management of multiple myeloma: a review. JAMA 327(5):464–477
Czabotar PE, Lessene G, Strasser A et al (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15(1):49–63
Da SI, de Castro LE, Pedroso AP et al (2020) Biochemical phenotyping of multiple myeloma patients at diagnosis reveals a disorder of mitochondrial complexes I and II and a Hartnup-like disturbance as underlying conditions, also influencing different stages of the disease. Sci Rep 10(1):21836
Dasgupta S, Soudry E, Mukhopadhyay N et al (2012) Mitochondrial DNA mutations in respiratory complex-I in never-smoker lung cancer patients contribute to lung cancer progression and associated with EGFR gene mutation. J Cell Physiol 227(6):2451–2460
Deblois G, Chahrour G, Perry MC et al (2010) Transcriptional control of the ERBB2 amplicon by ERRalpha and PGC-1beta promotes mammary gland tumorigenesis. Cancer Res 70(24):10277–10287
Desplanques G, Giuliani N, Delsignore R et al (2009) Impact of XIAP protein levels on the survival of myeloma cells. Haematologica 94(1):87–93
Dinndorf PA, Gootenberg J, Cohen MH et al (2007) FDA drug approval summary: pegaspargase (Oncaspar®) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist 12(8):991–998
Du C, Fang M, Li Y et al (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102(1):33–42
Fan J, Kamphorst JJ, Mathew R et al (2013) Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol 9:712
Fink EE, Mannava S, Bagati A et al (2016) Mitochondrial thioredoxin reductase regulates major cytotoxicity pathways of proteasome inhibitors in multiple myeloma cells. Leukemia 30(1):104–111
Fulda S, Vucic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 11(2):109–124
Gangemi S, Allegra A, Alonci A et al (2012) Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm Res 61(10):1063–1067
Gao P, Tchernyshyov I, Chang TC et al (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458(7239):762–765
Gasparre G, Kurelac I, Capristo M et al (2011) A mutation threshold distinguishes the antitumorigenic effects of the mitochondrial gene MTND1, an oncojanus function. Cancer Res 71(19):6220–6229
Gonsalves WI, Ramakrishnan V, Hitosugi T et al (2018) Glutamine-derived 2-hydroxyglutarate is associated with disease progression in plasma cell malignancies. JCI Insight. https://doi.org/10.1172/jci.insight.94543
Gonsalves WI, Jang JS, Jessen E et al (2020) In vivo assessment of glutamine anaplerosis into the TCA cycle in human pre-malignant and malignant clonal plasma cells. Cancer Metab 8(1):29
Graves PR, Aponte-Collazo LJ, Fennell EMJ et al (2019) Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem Biol 14(5):1020–1029
Greer YE, Porat-Shliom N, Nagashima K et al (2018) ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget 9(26):18454–18479
Hartmann FJ, Mrdjen D, McCaffrey E et al (2021) Single-cell metabolic profiling of human cytotoxic T cells. Nat Biotechnol 39(2):186–197
Hekmatshoar Y, Nakhle J, Galloni M et al (2018) The role of metabolism and tunneling nanotube-mediated intercellular mitochondria exchange in cancer drug resistance. Biochem J 475(14):2305–2328
Hoang PH, Cornish AJ, Chubb D et al (2020) Impact of mitochondrial DNA mutations in multiple myeloma. Blood Cancer J 10(5):46
Hu Y, Lu W, Chen G et al (2012) K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res 22(2):399–412
Huang Y, Si X, Shao M et al (2022) Rewiring mitochondrial metabolism to counteract exhaustion of CAR-T cells. J Hematol Oncol 15(1):38
Idelchik MDPS, Begley U, Begley TJ et al (2017) Mitochondrial ROS control of cancer. Semin Cancer Biol 47:57–66
Ishikawa K, Takenaga K, Akimoto M et al (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320(5876):661–664
Ishizawa J, Zarabi SF, Davis RE et al (2019) Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell 35(5):721–737
Jang JS, Li Y, Mitra AK et al (2019) Molecular signatures of multiple myeloma progression through single cell RNA-Seq. Blood Cancer J 9(1):2
Jia D, Lu M, Jung KH et al (2019) Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci USA 116(9):3909–3918
Joseph NS, Kaufman JL, Dhodapkar MV et al (2020) Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol 38(17):1928–1937
Katoh M, Nakagama H (2014) FGF receptors: cancer biology and therapeutics. Med Res Rev 34(2):280–300
Kawano Y, Moschetta M, Manier S et al (2015) Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev 263(1):160–172
Keats JJ, Fonseca R, Chesi M et al (2007) Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12(2):131–144
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337
Kumar S, Kaufman JL, Gasparetto C et al (2017) Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 130(22):2401–2409
Kumar SK, Harrison SJ, Cavo M et al (2020) Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 21(12):1630–1642
Kyle RA, Therneau TM, Rajkumar SV et al (2002) A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 346(8):564–569
Kyle RA, Remstein ED, Therneau TM et al (2007) Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 356(25):2582–2590
Le A, Lane AN, Hamaker M et al (2012) Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 15(1):110–121
Lebleu VS, O’Connell JT, Gonzalez HK et al (2014) PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16(10):992–1003
Lefebvre P, Benomar Y, Staels B (2010) Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab 21(11):676–683
Li F, Wang Y, Zeller KI et al (2005) Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 25(14):6225–6234
Li W, Qiu S, Chen J et al (2020) Chimeric antigen receptor designed to prevent ubiquitination and downregulation showed durable antitumor efficacy. Immunity 53(2):456–470
Liu VW, Shi HH, Cheung AN et al (2001) High incidence of somatic mitochondrial DNA mutations in human ovarian carcinomas. Cancer Res 61(16):5998–6001
Liu ZG, Tang B, Zeng Y et al (2016) Mitochondrial genome of a spontaneous multiple myeloma bone cancer model mouse C57BL/KaLwRij strain. Mitochondrial DNA A DNA Mapp Seq Anal 27(6):4071–4072
Maiso P, Huynh D, Moschetta M et al (2015) Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res 75(10):2071–2082
Maiuri MC, Kroemer G (2015) Essential role for oxidative phosphorylation in cancer progression. Cell Metab 21(1):11–12
Marlein CR, Piddock RE, Mistry JJ et al (2019) CD38-driven mitochondrial trafficking promotes bioenergetic plasticity in multiple myeloma. Cancer Res 79(9):2285–2297
Misund K, Keane N, Stein CK et al (2020) MYC dysregulation in the progression of multiple myeloma. Leukemia 34(1):322–326
Mizutani Y, Nakanishi H, Yamamoto K et al (2005) Downregulation of Smac/DIABLO expression in renal cell carcinoma and its prognostic significance. J Clin Oncol 23(3):448–454
Mizutani Y, Katsuoka Y, Bonavida B (2010) Prognostic significance of second mitochondria-derived activator of caspase (Smac/DIABLO) expression in bladder cancer and target for therapy. Int J Oncol 37(2):503–508
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13
Nakagawa Y, Abe S, Kurata M et al (2006) IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes. Am J Hematol 81(11):824–831
Neri A, Murphy JP, Cro L et al (1989) Ras oncogene mutation in multiple myeloma. J Exp Med 170(5):1715–1725
Nouri K, Feng Y, Schimmer AD (2020) Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis 11(10):841
Ogando J, Saez ME, Santos J et al (2019) PD-1 signaling affects cristae morphology and leads to mitochondrial dysfunction in human CD8(+) T lymphocytes. J Immunother Cancer 7(1):151
Patsoukis N, Bardhan K, Chatterjee P et al (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6:6692
Petersen CT, Hassan M, Morris AB et al (2018) Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3Kdelta inhibitors and VIP antagonists. Blood Adv 2(3):210–223
Petros JA, Baumann AK, Ruiz-Pesini E et al (2005) mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA 102(3):719–724
Pluta P, Cebula-Obrzut B, Ehemann V et al (2011) Correlation of Smac/DIABLO protein expression with the clinico-pathological features of breast cancer patients. Neoplasma 58(5):430–435
Porporato PE, Payen VL, Pérez-Escuredo J et al (2014) A mitochondrial switch promotes tumor metastasis. Cell Rep 8(3):754–766
Robak P, Drozdz I, Szemraj J et al (2018) Drug resistance in multiple myeloma. Cancer Treat Rev 70:199–208
Ronca R, Ghedini GC, Maccarinelli F et al (2020) FGF trapping inhibits multiple myeloma growth through c-Myc degradation-induced mitochondrial oxidative stress. Cancer Res 80(11):2340–2354
Roth KG, Mambetsariev I, Kulkarni P et al (2020) The mitochondrion as an emerging therapeutic target in cancer. Trends Mol Med 26(1):119–134
Sabharwal SS, Schumacker PT (2014) Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer 14(11):709–721
Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23(9):459–466
Schreier PH, Bankier AT, Roe BA et al (1981) Sequence and organization of the human mitochondrial genome. Nature 290(5806):457–465
Sedlackova L, Korolchuk VI (2019) Mitochondrial quality control as a key determinant of cell survival. Biochim Biophys Acta Mol Cell Res 1866(4):575–587
Soncini D, Minetto P, Martinuzzi C et al (2020) Amino acid depletion triggered by ʟ-asparaginase sensitizes MM cells to carfilzomib by inducing mitochondria ROS-mediated cell death. Blood Adv 4(18):4312–4326
Song IS, Kim HK, Lee SR et al (2013) Mitochondrial modulation decreases the bortezomib-resistance in multiple myeloma cells. Int J Cancer 133(6):1357–1367
Soriano GP, Besse L, Li N et al (2016) Proteasome inhibitor-adapted myeloma cells are largely independent from proteasome activity and show complex proteomic changes, in particular in redox and energy metabolism. Leukemia 30(11):2198–2207
Sullivan LB, Chandel NS (2014) Mitochondrial reactive oxygen species and cancer. Cancer Metab 2:17
Tanaka H, Matsumura I, Ezoe S et al (2002) E2F1 and c-Myc potentiate apoptosis through inhibition of NF-κB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell 9(5):1017–1029
Thompson RM, Dytfeld D, Reyes L et al (2017) Glutaminase inhibitor CB-839 synergizes with carfilzomib in resistant multiple myeloma cells. Oncotarget 8(22):35863–35876
Touzeau C, Ryan J, Guerriero J et al (2016) BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 30(3):761–764
Tu Y, He J, Liu H et al (2017) The imipridone ONC201 induces apoptosis and overcomes chemotherapy resistance by up-regulation of bim in multiple myeloma. Neoplasia 19(10):772–780
Urak R, Walter M, Lim L et al (2017) Ex vivo Akt inhibition promotes the generation of potent CD19CAR T cells for adoptive immunotherapy. J Immunother Cancer 5:26
Vafa O, Wade M, Kern S et al (2002) c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell 9(5):1031–1044
van de Donk N, Richardson PG, Malavasi F (2018) CD38 antibodies in multiple myeloma: back to the future. Blood 131(1):13–29
van de Donk N, Pawlyn C, Yong KL (2021a) Multiple myeloma. Lancet 397(10272):410–427
van de Donk NWCJ, Usmani SZ, Yong K (2021b) CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol 8(6):e446–e461
van Gisbergen MW, Voets AM, Starmans MH et al (2015) How do changes in the mtDNA and mitochondrial dysfunction influence cancer and cancer therapy? Challenges, opportunities and models. Mutat Res Rev Mutat Res 764:16–30
Vardhana SA, Hwee MA, Berisa M et al (2020) Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat Immunol 21(9):1022–1033
Villena JA (2015) New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J 282(4):647–672
Wang Q, Zhao D, Xian M et al (2020) MIF as a biomarker and therapeutic target for overcoming resistance to proteasome inhibitors in human myeloma. Blood 136(22):2557–2573
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35(8):427–433
Wise DR, Deberardinis RJ, Mancuso A et al (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 105(48):18782–18787
Xiang Y, Fang B, Liu Y et al (2020) SR18292 exerts potent antitumor effects in multiple myeloma via inhibition of oxidative phosphorylation. Life Sci 256:117971
Yu M, Wan Y, Zou Q (2010) Decreased copy number of mitochondrial DNA in Ewing’s sarcoma. Clin Chim Acta 411(9–10):679–683
Yu Y, Imrichova H, Wang H et al (2020) Disturbed mitochondrial dynamics in CD8(+) TILs reinforce T cell exhaustion. Nat Immunol 21(12):1540
Zhan X, Yu W, Franqui-Machin R et al (2017) Alteration of mitochondrial biogenesis promotes disease progression in multiple myeloma. Oncotarget 8(67):111213–111224
Zhang H, Li L, Chen Q et al (2018) PGC1beta regulates multiple myeloma tumor growth through LDHA-mediated glycolytic metabolism. Mol Oncol 12(9):1579–1595
Zheng W, O’Hear CE, Alli R et al (2018) PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia 32(5):1157–1167
Zhou L, Zhang Y, Leng Y et al (2019) The IAP antagonist birinapant potentiates bortezomib anti-myeloma activity in vitro and in vivo. J Hematol Oncol 12(1):25
Zu XL, Guppy M (2004) Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun 313(3):459–465
Acknowledgements
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
The Henan Province Young and Middle-aged Health Science and Technology Innovation Leading Talent Training Project (No. YXKC2020007); Zhongyuan Science and Technology Innovation Leadership Program (214200510023).
Author information
Authors and Affiliations
Contributions
Conception and design: BJ.F; manuscript writing: all authors; final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Institutional review board statement
This study is a literature review and does not require the informed consent of patients.
Informed consent statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: To correct the affiliation of one of the author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, Y., Wang, F. & Fang, B. Mitochondrial dysfunction and drug targets in multiple myeloma. J Cancer Res Clin Oncol 149, 8007–8016 (2023). https://doi.org/10.1007/s00432-023-04672-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04672-8