Abstract
Obesity represents a major health problem in the pediatric population with an increasing prevalence worldwide, associated with cardiovascular and metabolic disorders, and due to both genetic and environmental factors. Rare forms of obesity are mostly monogenic, and less frequently due to polygenic influence. Polygenic form of obesity is usually the common obesity with single gene variations exerting smaller impact on weight and is commonly non-syndromic.
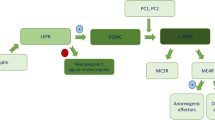
Non-syndromic monogenic obesity is associated with variants in single genes typically related to the hypothalamic leptin-melanocortin signalling pathway, which plays a key role in hunger and satiety regulation, thus body weight control. Patients with these genetic defects usually present with hyperphagia and early-onset severe obesity. Significant progress in genetic diagnostic testing has recently made for early identification of patients with genetic obesity, which guarantees prompt intervention in terms of therapeutic management of the disease.
What is Known: • Obesity represents a major health problem among children and adolescents, with an increasing prevalence worldwide, associated with cardiovascular disease and metabolic abnormalities, and it can be due to both genetic and environmental factors. • Non-syndromic monogenic obesity is linked to modifications in single genes usually involved in the hypothalamic leptin-melanocortin signalling pathway, which plays a key role in hunger and satiety regulation. | |
What is New: • The increasing understanding of rare forms of monogenic obesity has provided significant insights into the genetic causes of pediatric obesity, and our current knowledge of the various genes associated with childhood obesity is rapidly expanding. • A useful diagnostic algorithm for early identification of genetic obesity has been proposed, which can ensure a prompt intervention in terms of therapeutic management of the disease and an early prevention of the development of associated metabolic conditions. |


Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- AAP:
-
American Academy of Paediatrics
- AgRP:
-
Agouti-related protein
- ASIP:
-
Agouti signaling protein
- BBS:
-
Bardet-Biedl syndrome
- BDNF:
-
Brain-derived neurotrophic factor
- BDV:
-
Blakemore-Durmaz-Vasoleiou
- BMI:
-
Body mass index
- CDC:
-
Centres for Disease Control and Prevention
- ,CNVs:
-
Copy number variations
- CPE:
-
Carboxypeptidase E
- FTO:
-
Fat mass and obesity associated
- Gαs:
-
Stimulatory G-protein α subunit
- GPCR:
-
G protein coupled receptor
- GWAS:
-
Genome-Wide Association Studies
- LEPR:
-
Leptin receptor
- MC4R:
-
Melanocortin receptor type 4
- MSH:
-
Melanocyte-stimulating hormones
- NCOA1:
-
Nuclear receptor coactivator-1
- NGS:
-
Next generation sequencing
- NPY:
-
Neuropeptide Y
- NTRK2:
-
Neurotrophic tyrosine kinase receptor type 2 gene
- PCSK1:
-
Proprotein convertase subtilisin/kexin type 1
- POMC:
-
Proopiomelanocortin
- PWLS:
-
Prader-Willi-like syndrome
- PWS:
-
Prader-Willi syndrome
- SH2B1:
-
SH2B adapter protein 1
- SIM1:
-
Single-minded homolog 1
- SNPs:
-
Single nucleotide polymorphisms
- ,SRC-1:
-
Steroid receptor coactivator
- WES:
-
Whole exome sequencing
- WGS:
-
Whole genome sequence
- WHO:
-
World Health Organization
References
Mahmoud R, Kimonis V, Butler MG (2022) Genetics of obesity in humans: a clinical review. Int J Mol Sci 23:11005. https://doi.org/10.3390/ijms231911005
Woolford SJ, Sidell M, Li X et al (2021) Changes in body mass index among children and adolescents during the COVID-19 pandemic. JAMA 326:1434–1436. https://doi.org/10.1001/jama.2021.15036
Jebeile H, Kelly AS, O’Malley G, Baur LA (2022) Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol 10:351–365. https://doi.org/10.1016/S2213-8587(22)00047-X
Kaur Y, de Souza RJ, Gibson WT, Meyre D (2017) A systematic review of genetic syndromes with obesity. Obes Rev 18:603–634. https://doi.org/10.1111/obr.12531
Hinney A, Körner A, Fischer-Posovszky P (2022) The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat Rev Endocrinol 18:623–637. https://doi.org/10.1038/s41574-022-00716-0
Moreno LA (2018) Obesity: early severe obesity in children. Nat Rev Endocrinol 14:194–196. https://doi.org/10.1038/nrendo.2018.15
Body mass index-for-age (BMI-for-age). https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age. Accessed 17 Mar 2023
Growth Charts - Homepage. (2023) https://www.cdc.gov/growthcharts/index.htm. Accessed 17 Mar 2023
Weir CB, Jan A (2022) BMI classification percentile and cut off points. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Pedicelli S, Fintini D, Ravà L et al (2022) Prevalence of prediabetes in children and adolescents by class of obesity. Pediatr Obes 17:e12900. https://doi.org/10.1111/ijpo.12900
Nakhleh A, Sakhnini R, Furman E, Shehadeh N (2023) Cardiometabolic risk factors among children and adolescents with overweight and class 1 obesity: a cross-sectional study. Insights from stratification of Class 1 obesity. Front Endocrinol (Lausanne) 14:1108618. https://doi.org/10.3389/fendo.2023.1108618
Daneshzad E, Rostami S, Aghamahdi F et al (2022) Association of cardiometabolic risk factors with insulin resistance in overweight and obese children. BMC Endocr Disord 22:320. https://doi.org/10.1186/s12902-022-01245-7
Kumar S, Kelly AS (2017) Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc 92:251–265. https://doi.org/10.1016/j.mayocp.2016.09.017
Sohn YB (2022) Genetic obesity: an update with emerging therapeutic approaches. Ann Pediatr Endocrinol Metab 27:169–175. https://doi.org/10.6065/apem.2244188.094
Kleiser C, Schaffrath Rosario A, Mensink GBM et al (2009) Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS Study. BMC Public Health 9:46. https://doi.org/10.1186/1471-2458-9-46
Nelson MC, Gordon-Larsen P, Adair LS, Popkin BM (2005) Adolescent physical activity and sedentary behavior: patterning and long-term maintenance. Am J Prev Med 28:259–266. https://doi.org/10.1016/j.amepre.2004.12.006
Boone-Heinonen J, Gordon-Larsen P, Adair LS (2008) Obesogenic clusters: multidimensional adolescent obesity-related behaviors in the U.S. Ann Behav Med 36:217–230. https://doi.org/10.1007/s12160-008-9074-3
Geserick M, Vogel M, Gausche R et al (2018) Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med 379:1303–1312. https://doi.org/10.1056/NEJMoa1803527
Qiao Y, Ma J, Wang Y et al (2015) Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl 5:S74-79. https://doi.org/10.1038/ijosup.2015.23
Campbell MK (2016) Biological, environmental, and social influences on childhood obesity. Pediatr Res 79:205–211. https://doi.org/10.1038/pr.2015.208
Kleinendorst L, Abawi O, van der Voorn B et al (2020) Identifying underlying medical causes of pediatric obesity: results of a systematic diagnostic approach in a pediatric obesity center. PLoS One 15:e0232990. https://doi.org/10.1371/journal.pone.0232990
Styne DM, Arslanian SA, Connor EL et al (2017) Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 102:709–757. https://doi.org/10.1210/jc.2016-2573
Saeed S, Arslan M, Manzoor J et al (2020) Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes 69:1424–1438. https://doi.org/10.2337/db19-1238
Farooqi IS, O’Rahilly S (2008) Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4:569–577. https://doi.org/10.1038/ncpendmet0966
Schönauer R, Jin W, Findeisen C et al (2023) Monoallelic intragenic POU3F2 variants lead to neurodevelopmental delay and hyperphagic obesity, confirming the gene’s candidacy in 6q16.1 deletions. Am J Hum Genet 110:998–1007. https://doi.org/10.1016/j.ajhg.2023.04.010
Saeed S, Ning L, Badreddine A et al (2023) Biallelic mutations in P4HTM cause syndromic obesity. Diabetes db221017. https://doi.org/10.2337/db22-1017
Saeed S, Bonnefond A, Tamanini F et al (2018) Loss-of-function mutations in ADCY3 cause monogenic severe obesity. Nat Genet 50:175–179. https://doi.org/10.1038/s41588-017-0023-6
Littleton SH, Berkowitz RI, Grant SFA (2020) Genetic determinants of childhood obesity. Mol Diagn Ther 24:653–663. https://doi.org/10.1007/s40291-020-00496-1
Xia Q, Grant SFA (2013) The genetics of human obesity. Ann N Y Acad Sci 1281:178–190. https://doi.org/10.1111/nyas.12020
Bouchard C (2021) Genetics of obesity: what we have learned over decades of research. Obesity (Silver Spring) 29:802–820. https://doi.org/10.1002/oby.23116
Dayton K, Miller J (2018) Finding treatable genetic obesity: strategies for success. Curr Opin Pediatr 30:526–531. https://doi.org/10.1097/MOP.0000000000000641
Lyon HN, Hirschhorn JN (2005) Genetics of common forms of obesity: a brief overview. Am J Clin Nutr 82:215S–217S. https://doi.org/10.1093/ajcn/82.1.215S
Obradovic M, Sudar-Milovanovic E, Soskic S et al (2021) Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne) 12:585887. https://doi.org/10.3389/fendo.2021.585887
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395:763–770. https://doi.org/10.1038/27376
Singh RK, Kumar P, Mahalingam K (2017) Molecular genetics of human obesity: a comprehensive review. C R Biol 340:87–108. https://doi.org/10.1016/j.crvi.2016.11.007
Gregoric N, Groselj U, Bratina N et al (2021) Two cases with an early presented proopiomelanocortin deficiency-a long-term follow-up and systematic literature review. Front Endocrinol (Lausanne) 12:689387. https://doi.org/10.3389/fendo.2021.689387
Ericson MD, Lensing CJ, Fleming KA et al (2017) Bench-top to clinical therapies: a review of melanocortin ligands from 1954 to 2016. Biochim Biophys Acta Mol Basis Dis 1863:2414–2435. https://doi.org/10.1016/j.bbadis.2017.03.020
Frayling TM, Timpson NJ, Weedon MN et al (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–894. https://doi.org/10.1126/science.1141634
Chauhdary Z, Rehman K, Akash MSH (2021) The composite alliance of FTO locus with obesity-related genetic variants. Clin Exp Pharmacol Physiol 48:954–965. https://doi.org/10.1111/1440-1681.13498
Poitou C, Mosbah H, Clément K (2020) MECHANISMS IN ENDOCRINOLOGY: update on treatments for patients with genetic obesity. Eur J Endocrinol 183:R149–R166. https://doi.org/10.1530/EJE-20-0363
Cheon CK (2016) Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann Pediatr Endocrinol Metab 21:126–135. https://doi.org/10.6065/apem.2016.21.3.126
Goldstone AP, Holland AJ, Hauffa BP et al (2008) Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab 93:4183–4197. https://doi.org/10.1210/jc.2008-0649
Lloret-Linares C, Faucher P, Coupaye M et al (2013) Comparison of body composition, basal metabolic rate and metabolic outcomes of adults with Prader Willi syndrome or lesional hypothalamic disease, with primary obesity. Int J Obes (Lond) 37:1198–1203. https://doi.org/10.1038/ijo.2012.228
Bonnefond A, Raimondo A, Stutzmann F et al (2013) Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi-like features. J Clin Invest 123:3037–3041. https://doi.org/10.1172/JCI68035
Tsang SH, Aycinena ARP, Sharma T (2018) Ciliopathy: Bardet-Biedl syndrome. Adv Exp Med Biol 1085:171–174. https://doi.org/10.1007/978-3-319-95046-4_33
Kohlsdorf K, Nunziata A, Funcke J-B et al (2018) Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int J Obes (Lond) 42:1602–1609. https://doi.org/10.1038/s41366-018-0049-6
Heymsfield SB, Avena NM, Baier L et al (2014) Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity (Silver Spring) 22(Suppl 1):S1–S17. https://doi.org/10.1002/oby.20646
Beghini M, Brandt S, Körber I et al (2021) Serum IGF1 and linear growth in children with congenital leptin deficiency before and after leptin substitution. Int J Obes (Lond) 45:1448–1456. https://doi.org/10.1038/s41366-021-00809-2
Nordang GBN, Busk ØL, Tveten K et al (2017) Next-generation sequencing of the monogenic obesity genes LEP, LEPR, MC4R, PCSK1 and POMC in a Norwegian cohort of patients with morbid obesity and normal weight controls. Mol Genet Metab 121:51–56. https://doi.org/10.1016/j.ymgme.2017.03.007
Antunes H, Santos C, Carvalho S (2009) Serum leptin levels in overweight children and adolescents. Br J Nutr 101:1262–1266. https://doi.org/10.1017/S0007114508055682
Wasim M, Awan FR, Najam SS et al (2016) Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem Genet 54:565–572. https://doi.org/10.1007/s10528-016-9751-z
Montague CT, Farooqi IS, Whitehead JP et al (1997) Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903–908. https://doi.org/10.1038/43185
Wabitsch M, Funcke J-B, von Schnurbein J et al (2015) Severe early-onset obesity due to bioinactive leptin caused by a p. N103K mutation in the leptin gene. J Clin Endocrinol Metab 100:3227–3230. https://doi.org/10.1210/jc.2015-2263
Kleinendorst L, Abawi O, van der Kamp HJ et al (2020) Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. Eur J Endocrinol 182:47–56. https://doi.org/10.1530/EJE-19-0678
Clément K, Vaisse C, Lahlou N et al (1998) A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398–401. https://doi.org/10.1038/32911
Mazen IH, El-Gammal MA, Elaidy AA et al (2023) Congenital leptin and leptin receptor deficiencies in nine new families: identification of six novel variants and review of literature. Mol Genet Genomics 298:919–929. https://doi.org/10.1007/s00438-023-02025-1
Yeo GS, Farooqi IS, Aminian S et al (1998) A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20:111–112. https://doi.org/10.1038/2404
Farooqi IS, Keogh JM, Yeo GSH et al (2003) Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 348:1085–1095. https://doi.org/10.1056/NEJMoa022050
Hinney A, Volckmar A-L, Knoll N (2013) Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog Mol Biol Transl Sci 114:147–191. https://doi.org/10.1016/B978-0-12-386933-3.00005-4
Nalbantoğlu Ö, Hazan F, Acar S et al (2022) Screening of non-syndromic early-onset child and adolescent obese patients in terms of LEP, LEPR, MC4R and POMC gene variants by next-generation sequencing. J Pediatr Endocrinol Metab 35:1041–1050. https://doi.org/10.1515/jpem-2022-0027
Yeo GSH, Chao DHM, Siegert A-M et al (2021) The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol Metab 48:101206. https://doi.org/10.1016/j.molmet.2021.101206
Kühnen P, Krude H, Biebermann H (2019) Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends Mol Med 25:136–148. https://doi.org/10.1016/j.molmed.2018.12.002
Dempfle A, Hinney A, Heinzel-Gutenbrunner M et al (2004) Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J Med Genet 41:795–800. https://doi.org/10.1136/jmg.2004.018614
Lotta LA, Mokrosiński J, Mendes de Oliveira E et al (2019) Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 177:597–607.e9. https://doi.org/10.1016/j.cell.2019.03.044
Smith JS, Lefkowitz RJ, Rajagopal S (2018) Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 17:243–260. https://doi.org/10.1038/nrd.2017.229
Krude H, Biebermann H, Luck W et al (1998) Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157. https://doi.org/10.1038/509
Graves LE, Khouri JM, Kristidis P, Verge CF (2021) Proopiomelanocortin deficiency diagnosed in infancy in two boys and a review of the known cases. J Paediatr Child Health 57:484–490. https://doi.org/10.1111/jpc.15407
Candler T, Kühnen P, Prentice AM, Silver M (2019) Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front Neuroendocrinol 54:100773. https://doi.org/10.1016/j.yfrne.2019.100773
Farooqi IS, Volders K, Stanhope R et al (2007) Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab 92:3369–3373. https://doi.org/10.1210/jc.2007-0687
Jackson RS, Creemers JW, Ohagi S et al (1997) Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet 16:303–306. https://doi.org/10.1038/ng0797-303
Pépin L, Colin E, Tessarech M et al (2019) A new case of PCSK1 pathogenic variant with congenital proprotein convertase 1/3 deficiency and literature review. J Clin Endocrinol Metab 104:985–993. https://doi.org/10.1210/jc.2018-01854
Doche ME, Bochukova EG, Su H-W et al (2012) Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest 122:4732–4736. https://doi.org/10.1172/JCI62696
Li Z, Zhou Y, Carter-Su C et al (2007) SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol 21:2270–2281. https://doi.org/10.1210/me.2007-0111
Rui L (2014) SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J Diabetes 5:511–526. https://doi.org/10.4239/wjd.v5.i4.511
Bachmann-Gagescu R, Mefford HC, Cowan C et al (2010) Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med 12:641–647. https://doi.org/10.1097/GIM.0b013e3181ef4286
Mendes de Oliveira E, Keogh JM, Talbot F et al (2021) Obesity-associated GNAS mutations and the melanocortin pathway. N Engl J Med 385:1581–1592. https://doi.org/10.1056/NEJMoa2103329
Poon K-S, Tan KM (2022) Obesity-associated GNAS mutations and the melanocortin pathway. N Engl J Med 387:284. https://doi.org/10.1056/NEJMc2119110
Bosch E, Hebebrand M, Popp B et al (2021) BDV syndrome: an emerging syndrome with profound obesity and neurodevelopmental delay resembling Prader-Willi syndrome. J Clin Endocrinol Metab 106:3413–3427. https://doi.org/10.1210/clinem/dgab592
Durmaz A, Aykut A, Atik T et al (2021) A new cause of obesity syndrome associated with a mutation in the carboxypeptidase gene detected in three siblings with obesity, intellectual disability and hypogonadotropic hypogonadism. J Clin Res Pediatr Endocrinol 13:52–60. https://doi.org/10.4274/jcrpe.galenos.2020.2020.0101
Alsters SIM, Goldstone AP, Buxton JL et al (2015) Truncating homozygous mutation of carboxypeptidase E (CPE) in a morbidly obese female with type 2 diabetes mellitus, intellectual disability and hypogonadotrophic hypogonadism. PLoS One 10:e0131417. https://doi.org/10.1371/journal.pone.0131417
Holder JL, Butte NF, Zinn AR (2000) Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet 9:101–108. https://doi.org/10.1093/hmg/9.1.101
Michaud JL, Boucher F, Melnyk A et al (2001) Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet 10:1465–1473. https://doi.org/10.1093/hmg/10.14.1465
Stein CK, Stred SE, Thomson LL et al (1996) Interstitial 6q deletion and Prader-Willi-like phenotype. Clin Genet 49:306–310. https://doi.org/10.1111/j.1399-0004.1996.tb03794.x
Candelo E, Feinstein MM, Ramirez-Montaño D et al (2018) First case report of Prader-Willi-like syndrome in Colombia. Front Genet 9:98. https://doi.org/10.3389/fgene.2018.00098
El Khattabi L, Guimiot F, Pipiras E et al (2015) Incomplete penetrance and phenotypic variability of 6q16 deletions including SIM1. Eur J Hum Genet 23:1010–1018. https://doi.org/10.1038/ejhg.2014.230
Rosenfeld JA, Amrom D, Andermann E et al (2012) Genotype-phenotype correlation in interstitial 6q deletions: a report of 12 new cases. Neurogenetics 13:31–47. https://doi.org/10.1007/s10048-011-0306-5
D’Angelo CS, Kohl I, Varela MC et al (2013) Obesity with associated developmental delay and/or learning disability in patients exhibiting additional features: report of novel pathogenic copy number variants. Am J Med Genet A 161A:479–486. https://doi.org/10.1002/ajmg.a.35761
Blackburn PR, Sullivan AE, Gerassimou AG et al (2020) Functional analysis of the SIM1 variant p.G715V in 2 patients with obesity. J Clin Endocrinol Metab 105:dgz192. https://doi.org/10.1210/clinem/dgz192
Kasher PR, Schertz KE, Thomas M et al (2016) Small 6q16.1 deletions encompassing POU3F2 cause susceptibility to obesity and variable developmental delay with intellectual disability. Am J Hum Genet 98:363–372. https://doi.org/10.1016/j.ajhg.2015.12.014
Montagne L, Raimondo A, Delobel B et al (2014) Identification of two novel loss-of-function SIM1 mutations in two overweight children with developmental delay. Obesity (Silver Spring) 22:2621–2624. https://doi.org/10.1002/oby.20886
Izumi K, Housam R, Kapadia C et al (2013) Endocrine phenotype of 6q16.1-q21 deletion involving SIM1 and Prader-Willi syndrome-like features. Am J Med Genet A 161A:3137–3143. https://doi.org/10.1002/ajmg.a.36149
Vignoli A, Scornavacca GF, Peron A et al (2013) Interstitial 6q microdeletion syndrome and epilepsy: a new patient and review of the literature. Am J Med Genet A 161A:2009–2015. https://doi.org/10.1002/ajmg.a.35993
Wentzel C, Lynch SA, Stattin E-L et al (2010) Interstitial deletions at 6q14.1-q15 associated with obesity, developmental delay and a distinct clinical phenotype. Mol Syndromol 1:75–81. https://doi.org/10.1159/000314025
Wang J-C, Turner L, Lomax B, Eydoux P (2008) A 5-Mb microdeletion at 6q16.1-q16.3 with SIM gene deletion and obesity. Am J Med Genet A 146A:2975–2978. https://doi.org/10.1002/ajmg.a.32555
Bonaglia MC, Ciccone R, Gimelli G et al (2008) Detailed phenotype-genotype study in five patients with chromosome 6q16 deletion: narrowing the critical region for Prader-Willi-like phenotype. Eur J Hum Genet 16:1443–1449. https://doi.org/10.1038/ejhg.2008.119
Varela MC, Simões-Sato AY, Kim CA et al (2006) A new case of interstitial 6q16.2 deletion in a patient with Prader-Willi-like phenotype and investigation of SIM1 gene deletion in 87 patients with syndromic obesity. Eur J Med Genet 49:298–305. https://doi.org/10.1016/j.ejmg.2005.12.002
Faivre L, Cormier-Daire V, Lapierre JM et al (2002) Deletion of the SIM1 gene (6q16.2) in a patient with a Prader-Willi-like phenotype. J Med Genet 39:594–596. https://doi.org/10.1136/jmg.39.8.594
Cacciottolo TM, Henning E, Keogh JM et al (2022) Obesity due to steroid receptor coactivator-1 deficiency is associated with endocrine and metabolic abnormalities. J Clin Endocrinol Metab 107:e2532–e2544. https://doi.org/10.1210/clinem/dgac067
Yang Y, van der Klaauw AA, Zhu L et al (2019) Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nat Commun 10:1718. https://doi.org/10.1038/s41467-019-08737-6
Kempf E, Landgraf K, Stein R et al (2022) Aberrant expression of agouti signaling protein (ASIP) as a cause of monogenic severe childhood obesity. Nat Metab 4:1697–1712. https://doi.org/10.1038/s42255-022-00703-9
Malhotra S, Sivasubramanian R, Srivastava G (2021) Evaluation and management of early onset genetic obesity in childhood. J Pediatr Genet 10:194–204. https://doi.org/10.1055/s-0041-1731035
Huvenne H, Dubern B, Clément K, Poitou C (2016) Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts 9:158–173. https://doi.org/10.1159/000445061
Meienberg J, Bruggmann R, Oexle K, Matyas G (2016) Clinical sequencing: is WGS the better WES? Hum Genet 135:359–362. https://doi.org/10.1007/s00439-015-1631-9
Bamshad MJ, Ng SB, Bigham AW et al (2011) Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12:745–755. https://doi.org/10.1038/nrg3031
De Rosa MC, Chesi A, McCormack S et al (2019) Characterization of rare variants in MC4R in African American and Latino children with severe early-onset obesity. J Clin Endocrinol Metab 104:2961–2970. https://doi.org/10.1210/jc.2018-02657
Das Bhowmik A, Gupta N, Dalal A, Kabra M (2017) Whole exome sequencing identifies a homozygous nonsense variation in ALMS1 gene in a patient with syndromic obesity. Obes Res Clin Pract 11:241–246. https://doi.org/10.1016/j.orcp.2016.09.004
Girirajan S, Brkanac Z, Coe BP et al (2011) Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet 7:e1002334. https://doi.org/10.1371/journal.pgen.1002334
Zorn S, von Schnurbein J, Schirmer M et al (2022) Measuring hyperphagia in patients with monogenic and syndromic obesity. Appetite 178:106161. https://doi.org/10.1016/j.appet.2022.106161
Author information
Authors and Affiliations
Contributions
All authors contributed to the article conception and design. FM and FC conceptualized the study, FM, SLB, and MR drafted the initial manuscript, performed the literature search, and revised the final version of the manuscript. All authors supervised and critically reviewed the manuscript critically for important intellectual content, approved the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mainieri, F., La Bella, S., Rinaldi, M. et al. Rare genetic forms of obesity in childhood and adolescence, a comprehensive review of their molecular mechanisms and diagnostic approach. Eur J Pediatr 182, 4781–4793 (2023). https://doi.org/10.1007/s00431-023-05159-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05159-x