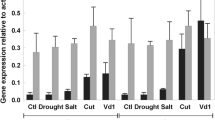
Abstract
Main conclusion
The Ve-resistance locus in tomato acts as a resilience gene by affecting both the stress/defense cascade and growth, constituting a signaling intercept with a competitive regulatory mechanism.
Abstract
For decades, the tomato Ve-gene has been recognized as a classical resistance R-gene, inherited as a dominant Mendelian trait and encoding a receptor protein that binds with a fungal effector to provide defense against Verticillium dahliae and V. albo-atrum. However, recent molecular studies suggest that the function and role(s) of the Ve-locus and the two proteins that it encodes are more complex than previously understood. This review summarizes both the background and recent molecular evidence and provides a reinterpretation of the function and role(s) of the Ve1- and Ve2-genes and proteins that better accommodates existing data. It is proposed that these two plasma membrane proteins interact to form a signaling intercept that directly links defense and growth. The induction of Ve1 by infection or wounding promotes growth but also downregulates Ve2 signaling, resulting in a decreased biosynthesis of PR proteins. In this context, the Ve1 R-gene acts as a Resilience gene rather than a Resistance gene, promoting taller more robust tomato plants with reduced symptoms (biotic and abiotic) and Verticillium concentration.



Similar content being viewed by others
Data availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed.
References
Bar M, Sharfman M, Avni A (2011) LeEix1 functions as a decoy receptor to attenuate LeEix2 signaling. Plant Signal Behav 6:455–457
Belostotsky DA, Sieburth LE (2009) Kill the messenger: mRNA decay and plant development. Curr Opin Plant Biol 121:96–102
Berne S, Javornik B (2016) Signalling crosstalk of plant defence responses to xylem-invading pathogens. In: Shanker A, Shanker C (eds) Abiotic and biotic stress in plants: recent advances and future perspectives. Intech Open, London, pp 1416–1435
Bishop CD, Cooper RM (1983) An ultrastructure study of root invasion in three vascular wilt diseases. Physiol Plant Pathol 22:15–27
Castroverde CDM (2016) Molecular biology of the tomato Ve gene family. Molecular and cellular biology. University of Guelph, Guelph, pp 1–205
Castroverde CDM, Nazar RN, Robb EJ (2016a) Verticillium Ave1 effector induces tomato defense gene expression independent of Ve1 prot. Plant Signal Behav 11:e1245254
Castroverde CDM, Nazar RN, Robb J (2016b) Verticillium Ave1 effector induces tomato defense gene expression independent of Ve1 protein. Plant Signal Behav 11:1–3
Castroverde CDM, Xu X, Nazar RN, Robb J (2017) Biotic factors that induce the tomato Ve1 R-gene. Plant Sci 265:61–69
Castroverde CDM, Morais de Avila LG, Romero VA, Blaya-Fernandez J, Nazar RN, Robb J (2019) Differential repression of the Ve R-genes in tomato. Plant Gene 18:100174
Chen P, Lee B, Robb J (2004) Tolerance to a non-host isolate of Verticillium dahliae in tomato. Physiol Mol Plant Pathol 64:283–291
Cheung AY, Qu L-J, Russinova E, Zhao Y, Zipfel C (2020) Update on receptors and signaling. Plant Physiol 182:1527–1530
Choi HW, Klessig DF (2016) DAMPs, Pamps and NAMPs inplant immunity. BMC Plant Biology 16:232–241
de Jonge R, van Esse HP, Maruthachalam K, Bolton MD, Santhanam P, Saber MK, Zhang Z, Usami T, Lievens B, Subbarao KV, Thomma BPHJ (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc Natl Acad Sci USA 109:5110–5115
Deance N, Sanchez V, Goffner D, Molina A (2013) Disease resistance or growth: the role of plant hormones in balancing immune responses and fitness costs. Front Plant Sci 4:155
Deng Y, Zhai K, Xie Z, Yang D, Zhu X, Liu J, Wang X, Qin P, Yang Y, Zhang G, Li Q, Zhang J, Wu S, Milazzo J, Mao B, Wang E, Xie H, Tharreau D, He Z (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 335:963–965
Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J (2008) Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol Plant 3:423–445
Dinkel H, Van Roey K, Michael S, Kumar M, Uyar B, Altenberg B, Milchevskaya V, Schneider M, Kühn H, Behrendt A, Dahl SL, Damerell V, Diebel S, Kalman S, Klein S, Knudsen AC, Mäder C, Merrill S, Staudt A, Thiel V, Welti L, Davey NE, Diella F, Gibson T (2016) ELM 2016–data update and new functionality of the eukaryotic linear motif resource. Nucleic Acids Res 44:D294-300
Diwan N, Fluhr R, Eshed Y, Zamir D, Tanksley SD (1999) Mapping of Ve in tomato: a gene conferring resistance to the broad-spectrum pathogen, Verticillium dahliae Kleb. race 1. Theor Appl Genet 98:315–319
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Flor HH (1942) Inheritance of pathogenicity in Melampsora lini. Phytopathology 32:653–669
Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9:275–296
Fradin EF, Thomma BPHJ (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol 7:71–86
Fradin EF, Zhang Z, Juarez-Ayala JC, Castroverde CDM, Nazar RN, Robb J, Liu C-M, Thomma BPHJ (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150:320–332
Fradin EF, Abd-El-Haliem A, Masini L, van den Berg GC, Joosten MH, Thomma BPHJ (2011) Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol 156:2255–2265
Fradin EF, Zhang Z, Rovenich H, Song Y, Liebrand TW, Masini L, van den Berg GC, Joosten MH, Thomma BPHJ (2014) Functional analysis of the tomato immune receptor Ve1 through domain swaps with its non-functional homologue Ve2. PLoS ONE 9:e88208
Franci LJ, Wheeler TA (1993) Interaction of plant-parasitic nematodes with wilt-inducing fungi. In: Khan W (ed) Nematode interactions. Springer, New York, pp 79–103
Freeman BC, Beattie GA (2008) An overview of plant defenses against pathogens and herbivores. In: The plant health instructor. Iowa State University, Ames
Gao Q-M, Zhu S, Kachroo P, Kachroo A (2015) Signal regulators of systemic acquired resistance. Front Plant Sci 6:228
Genoud T, Métraux J-P (1999) Crosstalk in plant cell signaling: structure and function of the genetic network. Trends Plant Sci 4:503–507
Gold J, Robb J (1995) The role of the coating response in Craigella tomatoes infected with Verticillium dahliae, races 1 and 2. Physiol Mol Plant Pathol 47:141–157
He M, He C-Q, Ding NZ (2018a) Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front Plant Sci 9:1771
He Y, Zhou J, Shan L, Meng X (2018b) Plant cell surface receptor-mediated signaling—a common theme amid diversity. J Cell Sci 131:jcs209353
Heath M (2000) Hypersensitive response-related death. Plant Mol Biol 44:321–334
Heinz R, Lee SW, Saparno A, Nazar RN, Robb J (1998) Cyclical systemic colonization in Verticillium-infected tomato. Physiol Mol Plant Pathol 52:385–396
Imran QM, Yun B-W (2020) Pathogen-induced defense strategies in plants. J Crop Sci Biotech 23:97–105
Ingvardsen C, Veierskov B (2001) Ubiquitin- and proteasome-dependent proteolysis in plants. Physiol Plant 112:451–459
Irani NG, Russinova E (2009) Receptor endocytosis and signaling in plants. Curr Opin Plant Biol 12:653–659
Jiang D, Liang J, Noble PW (2007) Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23:435–461
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Karasov TL, Chae E, Herman JJ, Bergelson J (2017) Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 29:666–680
Kawchuk LM, Lynch DR, Hachey J, Bains PS, Kulcsar F (1994) Identification of a codominant amplified polymorphic DNA marker linked to the verticillium wilt resistance gene in tomato. Theor Appl Genet 89:661–664
Kawchuk L, Hachey J, Lynch DR, Klcsar F, van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, Fischer R, Prufer D (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA 98:6511–6515
Ku Y-S, Sintaha M, Cheung M-Y, Lam H-M (2018) Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci 19:3206
Liang FS, Kong FN, Zhou CJ, Cao PX, Ye CJ, Wang B (2005) Cloning and characterization of a non-TIR-NBS-LRR type disease resistance gene analogue from peach. DNA Seq 16:103–110
Mace ME, Bell AA, Beckman CH (1981) Fungal wilt diseases of plants. Academic Press, New York
Mackey M, Kurosky A, Robb EJ, Nazar RN (2018) A graft mimic strategy for verticillium resistance in Tomato. Mol Biotechnol 60:665–669
McAdam SAM, Brodribb TJ, Ross JJ (2016) Shoot-derived abscisic acid promotes root growth. Plant Cell Environ 39:652–659
Mulligan RM, Chory J, Ecker JR (1997) Signaling in plants. Proc Natl Acad Sci USA 94:2793–2795
Nazar RN, Robb J (2019) Signaling crosstalk in the Ve-resistance locus of tomato. Atlas of Science
Nazar RN, Castroverde CDM, Xu X, Kurosky A, Robb J (2018a) Wounding induces Tomato Ve1 R-gene expression, 2nd edon. In: Global conference on plant science and molecular biology
Nazar RN, Xu X, Kurosky A, Robb J (2018b) Antagonistic function of the Ve R-genes in tomato. Plant Mol Biol 98:67–79
Nazar RN, Xu X, Shittu HO, Kurosky A, Robb EJ (2018c) Tomato Ve resistance locus; defense or growth. Planta 247:1339–1350
Nazar RN, Xu X, Lee SW, Robb J (2020) The Ve-resistance locus, a plant signaling intercept. Planta 252:7–11
Nishad R, Ahmed T, Rahman VJ, Kareem A (2020) Modulation of plant defense system in response to microbial interactions. Front Microbiol 11:1298
Pegg GF, Brady BL (2002) Verticillium wilts. CABI Publishing, Wallingford, Oxon
Powelson ML, Rowe RC (1993) Biology and management of early dying of potatoes. Annu Rev Phytopathol 31:111–126
Preston GM (2000) Pseudomonas syringae pv. tomato: the right pathogen, of the right plant, at the right time. Mol Plant Pathol 1:263–275
Robb EJ, Powell DA, Street PFS (1989) Vascular coating: a barrier to colonization by the pathogen in Verticillium wilt of tomato. Can J Bot 67:600–607
Robb EJ, Shittu HO, Soman KV, Kurosky A, Nazar RN (2012) Elevated defense protein fails to protect tomato against Verticillium dahliae. Planta 236:623–633
Ruthardt N, Fischer R, Emans N, Kawchuk LM (2007) Tomato protein of the resistance gene Ve2 to verticillium wilt [Verticillium spp.] is located in the endoplasmic reticulum. Can J Plant Pathol 29:3–8
Schaible L, Cannon OS, Waddoups V (1951) Inheritance of resistance to Verticillium wilt in a tomato cross. Phytopathology 41:986–990
Sharfman M, Bar M, Ehrlich M, Schuster S, Melech-Bonfil S, Ezer R, Sessa S, Avni A (2011) Endosomal signaling of the tomato leucine-rich repeat receptor-like protein LeEix2. Plant J 68:418–423
Song Y, Zhang Z, Seidl MF, Majer A, Jakse JJ, Javornik B, Thomma BPHJ (2017) Broad taxonomic characterization of Verticillium wilt resistance genes reveals an ancient origin of the tomato Ve1 immune receptor. Mol. Plant Pathol. 18:195–201
Street PFS, Robb J, Ellis BE (1986) Secretion of vascular coating components by xylem parenchyma cells of tomatoes infected with Verticillium albo-atrum. Protoplasma 132:1–11
Taylor JE, McAinsh MR (2004) Signalling crosstalk in plants: emerging issues. J Exptl Bot 55:147–149
Thomma BPHJ, Van Esse HP, Crous PW, De Wit PJGM (2005) Cladosporium fulvum (syn. Passalora fulva), a highly specialized plant pathogen as a model for functional studies on plant pathogenic Mycosphaerellaceae. Mol Plant Pathol 6:361–489
Thomma BPHJ, Nurnberger T, Joosten MH (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23:4–15
van Esse HP, Fradin EF, de Groot PJ, de Wit PJGM, Thomma BPHJ (2009) Tomato transcriptional responses to a foliar and a vascular fungal pathogen are distinct. Mol Plant Microbe Interact 22:245–258
Van Ooijin G, Van der Burg HA, Cornelissen BJC, Takken FLW (2007) Structure and function of resistance proteins in Solanaceous plants. Ann Rev Phytopathol 45:43–72
Vert G, Chory J (2011) Crosstalk in cellular signaling: background noise or the real thing? Dev. Cell 21:985–991
Wang G, Ellendorff U, Kemp B, Mansfield JW, Forsyth A, Mitchell K, Bastas K, Liu C-M, Woods-Tör A, Zipfel C, de Wit PJGM, Jones JDG, Tör M, Thomma BPHJ (2008) A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol 147:503–517
Warren RF, Henk A, Mowery P, Holub E, Innes RW (1998) A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10:1439–1462
Xu X, Robb J, Nazar RN (2017) A “whole pot” strategy for root growth quantification or microbiota-root interaction studies. Soil Biol Biochem 111:154–156
Zhang Z, van Esse HP, van Damme M, Fradin EF, Liu C-M, Thomma BPHJ (2013) Ve1-mediated resistance against Verticillium does not involve a hypersensitive response in Arabidopsis. Mol Plant Pathol 14:719–727
Zhang Z, Song Y, Liu CM, Thomma B (2014) Mutational analysis of the Ve1 immune receptor that mediates Verticillium resistance in tomato. PLoS ONE 9:e99511
Zhang G, Zhao F, Chen L, Pan Y, Sun L, Bao N, Zhang T, Cui C-X, Qiu Z, Zhang Y, Yang L, Xu L (2019) Jasmonate-mediated wound signalling promotes plant regeneration. Nat Plants 5:491–497
Zhou W, Lozano-Torres JL, Blilou I, Zhang X, Zhai Q, Smant G, Scheres B (2019) A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 177:942–956
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Robb, E.J., Nazar, R.N. Tomato Ve-resistance locus: resilience in the face of adversity?. Planta 254, 126 (2021). https://doi.org/10.1007/s00425-021-03783-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03783-1