Abstract
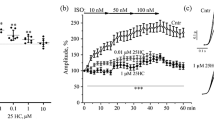
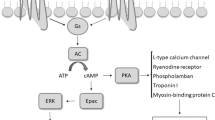
25-Hydroxycholesterol (25HC) is a biologically active oxysterol, whose production greatly increases during inflammation by macrophages and dendritic cells. The inflammatory reactions are frequently accompanied by changes in heart regulation, such as blunting of the cardiac β-adrenergic receptor (AR) signaling. Here, the mechanism of 25HC-dependent modulation of responses to β-AR activation was studied in the atria of mice. 25HC at the submicromolar levels decreased the β-AR-mediated positive inotropic effect and enhancement of the Ca2+ transient amplitude, without changing NO production. Positive inotropic responses to β1-AR (but not β2-AR) activation were markedly attenuated by 25HC. The depressant action of 25HC on the β1-AR-mediated responses was prevented by selective β3-AR antagonists as well as inhibitors of Gi protein, Gβγ, G protein-coupled receptor kinase 2/3, or β-arrestin. Simultaneously, blockers of protein kinase D and C as well as a phosphodiesterase inhibitor did not preclude the negative action of 25HC on the inotropic response to β-AR activation. Thus, 25HC can suppress the β1-AR-dependent effects via engaging β3-AR, Gi protein, Gβγ, G protein-coupled receptor kinase, and β-arrestin. This 25HC-dependent mechanism can contribute to the inflammatory-related alterations in the atrial β-adrenergic signaling.







Similar content being viewed by others
Data availability
All mentioned data are represented in the main manuscript figures and supplementary figure. Other additional data will be made available on reasonable request.
Abbreviations
- AR:
-
Adrenoceptor
- 25HC:
-
25-Hydroxycholesterol
- GRK:
-
G protein-coupled receptor kinase
- NO:
-
Nitric oxide
- ISO:
-
Isoproterenol
- PKC:
-
Protein kinase C
- PKD:
-
Protein kinase D
- PDE:
-
Phosphodiesterase
References
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL (2020) Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 17:269–285. https://doi.org/10.1038/s41569-019-0315-x
Agarwal SR, Sherpa RT, Moshal KS, Harvey RD (2022) Compartmentalized cAMP signaling in cardiac ventricular myocytes. Cell Signal 89:110172. https://doi.org/10.1016/j.cellsig.2021.110172
Algoet M, Janssens S, Himmelreich U, Gsell W, Pusovnik M, Van den Eynde J, Oosterlinck W (2023) Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med 33:357–366. https://doi.org/10.1016/j.tcm.2022.02.005
Arioglu-Inan E, Kayki-Mutlu G, Michel MC (2019) Cardiac beta(3) -adrenoceptors-a role in human pathophysiology? Br J Pharmacol 176:2482–2495. https://doi.org/10.1111/bph.14635
Baker JG (2010) The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol 160:1048–1061. https://doi.org/10.1111/j.1476-5381.2010.00754.x
Balligand JL (2016) Cardiac salvage by tweaking with beta-3-adrenergic receptors. Cardiovasc Res 111:128–133. https://doi.org/10.1093/cvr/cvw056
Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M (2010) Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem 285:5674–5682. https://doi.org/10.1074/jbc.M109.066456
Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW (2009) 25-Hydroxycholesterol secreted by macrophages in response to toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci U S A 106:16764–16769. https://doi.org/10.1073/pnas.0909142106
Beautrait A, Paradis JS, Zimmerman B, Giubilaro J, Nikolajev L, Armando S, Kobayashi H, Yamani L, Namkung Y, Heydenreich FM, Khoury E, Audet M, Roux PP, Veprintsev DB, Laporte SA, Bouvier M (2017) A new inhibitor of the beta-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat Commun 8:15054. https://doi.org/10.1038/ncomms15054
Breit A, Lagace M, Bouvier M (2004) Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J Biol Chem 279:28756–28765. https://doi.org/10.1074/jbc.M313310200
Campbell AP, Smrcka AV (2018) Targeting G protein-coupled receptor signalling by blocking G proteins. Nat Rev Drug Discov 17:789–803. https://doi.org/10.1038/nrd.2018.135
Cannavo A, Koch WJ (2017) Targeting beta3-adrenergic receptors in the heart: selective agonism and beta-blockade. J Cardiovasc Pharmacol 69:71–78. https://doi.org/10.1097/FJC.0000000000000444
Cannavo A, Liccardo D, Koch WJ (2013) Targeting cardiac beta-adrenergic signaling via GRK2 inhibition for heart failure therapy. Front Physiol 4:264. https://doi.org/10.3389/fphys.2013.00264
Cernecka H, Sand C, Michel MC (2014) The odd sibling: features of beta3-adrenoceptor pharmacology. Mol Pharmacol 86:479–484. https://doi.org/10.1124/mol.114.092817
Chen H, Cao N, Wang L, Wu Y, Wei H, Li Y, Zhang Y, Zhang S, Liu H (2021) Biased activation of beta(2)-AR/Gi/GRK2 signal pathway attenuated beta(1)-AR sustained activation induced by beta(1)-adrenergic receptor autoantibody. Cell Death Discov 7:340. https://doi.org/10.1038/s41420-021-00735-2
Christ T, Molenaar P, Klenowski PM, Ravens U, Kaumann AJ (2011) Human atrial beta(1L)-adrenoceptor but not beta(3)-adrenoceptor activation increases force and Ca(2+) current at physiological temperature. Br J Pharmacol 162:823–839. https://doi.org/10.1111/j.1476-5381.2010.00996.x
Cuello F, Bardswell SC, Haworth RS, Yin X, Lutz S, Wieland T, Mayr M, Kentish JC, Avkiran M (2007) Protein kinase D selectively targets cardiac troponin I and regulates myofilament Ca2+ sensitivity in ventricular myocytes. Circ Res 100:864–873. https://doi.org/10.1161/01.RES.0000260809.15393.fa
Cyster JG, Dang EV, Reboldi A, Yi T (2014) 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 14:731–743. https://doi.org/10.1038/nri3755
Dalal S, Connelly B, Singh M, Singh K (2018) NF2 signaling pathway plays a pro-apoptotic role in beta-adrenergic receptor stimulated cardiac myocyte apoptosis. PLoS One 13:e0196626. https://doi.org/10.1371/journal.pone.0196626
Devic E, Xiang Y, Gould D, Kobilka B (2001) Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol 60:577–583
Diczfalusy U, Olofsson KE, Carlsson AM, Gong M, Golenbock DT, Rooyackers O, Flaring U, Bjorkbacka H (2009) Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J Lipid Res 50:2258–2264. https://doi.org/10.1194/jlr.M900107-JLR200
Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D (2014) beta-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol 4:396. https://doi.org/10.3389/fphys.2013.00396
Ferrero KM, Koch WJ (2022) GRK2 in cardiovascular disease and its potential as a therapeutic target. J Mol Cell Cardiol 172:14–23. https://doi.org/10.1016/j.yjmcc.2022.07.008
Gater DL, Saurel O, Iordanov I, Liu W, Cherezov V, Milon A (2014) Two classes of cholesterol binding sites for the beta2AR revealed by thermostability and NMR. Biophys J 107:2305–2312. https://doi.org/10.1016/j.bpj.2014.10.011
Gc JB, Chen J, Pokharel SM, Mohanty I, Mariasoosai C, Obi P, Panipinto P, Bandyopadhyay S, Bose S, Natesan S (2023) Molecular basis for the recognition of 24-(S)-hydroxycholesterol by integrin alphavbeta3. Sci Rep 13:9166. https://doi.org/10.1038/s41598-023-36040-4
Guimond J, Mamarbachi AM, Allen BG, Rindt H, Hebert TE (2005) Role of specific protein kinase C isoforms in modulation of beta1- and beta2-adrenergic receptors. Cell Signal 17:49–58. https://doi.org/10.1016/j.cellsig.2004.05.012
Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF (1989) Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A 86:6753–6757. https://doi.org/10.1073/pnas.86.17.6753
Hagiwara S, Iwasaka H, Maeda H, Noguchi T (2009) Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist, has protective effects in an LPS-induced systemic inflammation model. Shock 31:515–520. https://doi.org/10.1097/SHK.0b013e3181863689
Ippolito M, Benovic JL (2021) Biased agonism at beta-adrenergic receptors. Cell Signal 80:109905. https://doi.org/10.1016/j.cellsig.2020.109905
Ishikawa T, Kume H, Kondo M, Ito Y, Yamaki K, Shimokata K (2003) Inhibitory effects of interferon-gamma on the heterologous desensitization of beta-adrenoceptors by transforming growth factor-beta 1 in tracheal smooth muscle. Clin Exp Allergy 33:808–815. https://doi.org/10.1046/j.1365-2222.2003.01681.x
Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, Malhotra V (1999) Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell 98:59–68. https://doi.org/10.1016/S0092-8674(00)80606-6
Karam S, Margaria JP, Bourcier A, Mika D, Varin A, Bedioune I, Lindner M, Bouadjel K, Dessillons M, Gaudin F, Lefebvre F, Mateo P, Lechene P, Gomez S, Domergue V, Robert P, Coquard C, Algalarrondo V, Samuel JL et al (2020) Cardiac overexpression of PDE4B blunts beta-adrenergic response and maladaptive remodeling in heart failure. Circulation 142:161–174. https://doi.org/10.1161/CIRCULATIONAHA.119.042573
Kayki-Mutlu G, Karaomerlioglu I, Arioglu-Inan E, Altan VM (2019) Beta-3 adrenoceptors: a potential therapeutic target for heart disease. Eur J Pharmacol 858:172468. https://doi.org/10.1016/j.ejphar.2019.172468
Khamssi M, Brodde OE (1990) The role of cardiac beta1- and beta2-adrenoceptor stimulation in heart failure. J Cardiovasc Pharmacol 16(Suppl 5):S133–S137
Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M (2011) The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes. PLoS One 6:e16631. https://doi.org/10.1371/journal.pone.0016631
Liu Y, Wei Z, Ma X, Yang X, Chen Y, Sun L, Ma C, Miao QR, Hajjar DP, Han J, Duan Y (2018) 25-Hydroxycholesterol activates the expression of cholesterol 25-hydroxylase in an LXR-dependent mechanism. J Lipid Res 59:439–451. https://doi.org/10.1194/jlr.M080440
MacNeil BJ, Jansen AH, Greenberg AH, Nance DM (1996) Activation and selectivity of splenic sympathetic nerve electrical activity response to bacterial endotoxin. Am J Physiol 270:R264–R270. https://doi.org/10.1152/ajpregu.1996.270.1.R264
Mahmood A, Ahmed K, Zhang Y (2022) Beta-adrenergic receptor desensitization/down-regulation in heart failure: a friend or foe? Front Cardiovasc Med 9:925692. https://doi.org/10.3389/fcvm.2022.925692
Massion PB, Pelat M, Belge C, Balligand JL (2005) Regulation of the mammalian heart function by nitric oxide. Comp Biochem Physiol A Mol Integr Physiol 142:144–150. https://doi.org/10.1016/j.cbpb.2005.05.048
Matsuda N, Hattori Y, Akaishi Y, Suzuki Y, Kemmotsu O, Gando S (2000) Impairment of cardiac beta-adrenoceptor cellular signaling by decreased expression of G(s alpha) in septic rabbits. Anesthesiology 93:1465–1473. https://doi.org/10.1097/00000542-200012000-00019
Mira Hernandez J, Ko CY, Mandel AR, Shen EY, Baidar S, Christensen AR, Hellgren K, Morotti S, Martin JL, Hegyi B, Bossuyt J, Bers DM (2023) Cardiac protein kinase D1 ablation alters the myocytes beta-adrenergic response. J Mol Cell Cardiol 180:33–43. https://doi.org/10.1016/j.yjmcc.2023.05.001
Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M (2006) Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98:226–234. https://doi.org/10.1161/01.RES.0000200178.34179.93
Mukai H, Munekata E, Higashijima T (1992) G protein antagonists. A novel hydrophobic peptide competes with receptor for G protein binding. J Biol Chem 267:16237–16243
Neely OC, Domingos AI, Paterson DJ (2021) Macrophages can drive sympathetic excitability in the early stages of hypertension. Front Cardiovasc Med 8:807904. https://doi.org/10.3389/fcvm.2021.807904
Neumann J, Boknik P, Matherne GP, Lankford A, Schmitz W (2003) Pertussis toxin sensitive and insensitive effects of adenosine and carbachol in murine atria overexpressing A(1)-adenosine receptors. Br J Pharmacol 138:209–217. https://doi.org/10.1038/sj.bjp.0705012
Nso N, Bookani KR, Metzl M, Radparvar F (2021) Role of inflammation in atrial fibrillation: a comprehensive review of current knowledge. J Arrhythm 37:1–10. https://doi.org/10.1002/joa3.12473
Odnoshivkina YG, Petrov AM (2021) The role of neuro-cardiac junctions in sympathetic regulation of the heart. J Evol Biochem Physiol 57:527–541. https://doi.org/10.1134/s0022093021030078
Odnoshivkina UG, Petrov AM (2023) Immune oxysterol downregulates the atrial inotropic response to β-adrenergic receptor stimulation: the role of liver X receptors and lipid raft stability. J Evol Biochem Physiol 58:S1–S12. https://doi.org/10.1134/s0022093022070018
Odnoshivkina UG, Sytchev VI, Nurullin LF, Giniatullin AR, Zefirov AL, Petrov AM (2015) β2-adrenoceptor agonist-evoked reactive oxygen species generation in mouse atria: implication in delayed inotropic effect. Eur J Pharmacol 765:140–153. https://doi.org/10.1016/j.ejphar.2015.08.020
Odnoshivkina YG, Sytchev VI, Petrov AM (2017) Cholesterol regulates contractility and inotropic response to beta2-adrenoceptor agonist in the mouse atria: involvement of Gi-protein-Akt-NO-pathway. J Mol Cell Cardiol 107:27–40. https://doi.org/10.1016/j.yjmcc.2016.05.001
Odnoshivkina UG, Sytchev VI, Starostin O, Petrov AM (2019) Brain cholesterol metabolite 24-hydroxycholesterol modulates inotropic responses to beta-adrenoceptor stimulation: The role of NO and phosphodiesterase. Life Sci 220:117–126. https://doi.org/10.1016/j.lfs.2019.01.054
Odnoshivkina UG, Kuznetsova EA, Petrov AM (2022) 25-Hydroxycholesterol as a signaling molecule of the nervous system. Biochemistry (Mosc) 87:524–537. https://doi.org/10.1134/S0006297922060049
Okyere AD, Song J, Patwa V, Carter RL, Enjamuri N, Lucchese AM, Ibetti J, de Lucia C, Schumacher SM, Koch WJ, Cheung JY, Benovic JL, Tilley DG (2023) Pepducin ICL1-9-mediated beta2-adrenergic receptor-dependent cardiomyocyte contractility occurs in a G(i) protein/ROCK/PKD-sensitive manner. Cardiovasc Drugs Ther 37:245–256. https://doi.org/10.1007/s10557-021-07299-4
Pardini BJ, Jones SB, Filkins JP (1983) Cardiac and splenic norepinephrine turnovers in endotoxic rats. Am J Physiol 245:H276–H283. https://doi.org/10.1152/ajpheart.1983.245.2.H276
Penela P, Ribas C, Sanchez-Madrid F, Mayor F Jr (2019) G protein-coupled receptor kinase 2 (GRK2) as a multifunctional signaling hub. Cell Mol Life Sci 76:4423–4446. https://doi.org/10.1007/s00018-019-03274-3
Pfleger J, Gresham K, Koch WJ (2019) G protein-coupled receptor kinases as therapeutic targets in the heart. Nat Rev Cardiol 16:612–622. https://doi.org/10.1038/s41569-019-0220-3
Poli G, Leoni V, Biasi F, Canzoneri F, Risso D, Menta R (2022) Oxysterols: from redox bench to industry. Redox Biol 49:102220. https://doi.org/10.1016/j.redox.2021.102220
Pott C, Brixius K, Bundkirchen A, Bolck B, Bloch W, Steinritz D, Mehlhorn U, Schwinger RH (2003) The preferential beta3-adrenoceptor agonist BRL 37344 increases force via beta1-/beta2-adrenoceptors and induces endothelial nitric oxide synthase via beta3-adrenoceptors in human atrial myocardium. Br J Pharmacol 138:521–529. https://doi.org/10.1038/sj.bjp.0705065
Ramesh P, Yeo JL, Brady EM, McCann GP (2022) Role of inflammation in diabetic cardiomyopathy. Ther Adv Endocrinol Metab 13:20420188221083530. https://doi.org/10.1177/20420188221083530
Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M (2008) Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J 27:384–393. https://doi.org/10.1038/sj.emboj.7601968
Rozengurt E (2011) Protein kinase D signaling: multiple biological functions in health and disease. Physiology (Bethesda) 26:23–33. https://doi.org/10.1152/physiol.00037.2010
Rudokas MW, Post JP, Sataray-Rodriguez A, Sherpa RT, Moshal KS, Agarwal SR, Harvey RD (2021) Compartmentation of beta(2) -adrenoceptor stimulated cAMP responses by phosphodiesterase types 2 and 3 in cardiac ventricular myocytes. Br J Pharmacol 178:1574–1587. https://doi.org/10.1111/bph.15382
Sanchez-Alonso JL, Fedele L, Copier JS, Lucarelli C, Mansfield C, Judina A, Houser SR, Brand T, Gorelik J (2023) Functional LTCC-beta(2)AR complex needs caveolin-3 and is disrupted in heart failure. Circ Res 133:120–137. https://doi.org/10.1161/CIRCRESAHA.123.322508
Shi Q, Li M, Mika D, Fu Q, Kim S, Phan J, Shen A, Vandecasteele G, Xiang YK (2017) Heterologous desensitization of cardiac beta-adrenergic signal via hormone-induced betaAR/arrestin/PDE4 complexes. Cardiovasc Res 113:656–670. https://doi.org/10.1093/cvr/cvx036
Simsek Papur O, Sun A, Glatz JFC, Luiken J, Nabben M (2018) Acute and chronic effects of protein kinase-D signaling on cardiac energy metabolism. Front Cardiovasc Med 5:65. https://doi.org/10.3389/fcvm.2018.00065
Sytchev VI, Odnoshivkina YG, Ursan RV, Petrov AM (2017) Oxysterol, 5alpha-cholestan-3-one, modulates a contractile response to beta2-adrenoceptor stimulation in the mouse atria: Involvement of NO signaling. Life Sci 188:131–140. https://doi.org/10.1016/j.lfs.2017.09.006
Thangamalai R, Kandasamy K, Sukumarn SV, Reddy N, Singh V, Choudhury S, Parida S, Singh TU, Boobalan R, Mishra SK (2014) Atorvastatin prevents sepsis-induced downregulation of myocardial beta1-adrenoceptors and decreased cAMP response in mice. Shock 41:406–412. https://doi.org/10.1097/SHK.0000000000000138
Ursan R, Odnoshivkina UG, Petrov AM (2019) Membrane cholesterol oxidation downregulates atrial beta-adrenergic responses in ROS-dependent manner. Cell Signal 67:109503. https://doi.org/10.1016/j.cellsig.2019.109503
Vielma AZ, Leon L, Fernandez IC, Gonzalez DR, Boric MP (2016) Nitric oxide synthase 1 modulates basal and beta-adrenergic-stimulated contractility by rapid and reversible redox-dependent S-nitrosylation of the heart. PLoS One 11:e0160813. https://doi.org/10.1371/journal.pone.0160813
Volovyk ZM, Wolf MJ, Prasad SV, Rockman HA (2006) Agonist-stimulated beta-adrenergic receptor internalization requires dynamic cytoskeletal actin turnover. J Biol Chem 281:9773–9780. https://doi.org/10.1074/jbc.M511435200
Wang Y, Wang Y, Yang D, Yu X, Li H, Lv X, Lu D, Wang H (2015) beta(1)-adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IkappaBalpha phosphorylation. Crit Care 19:76. https://doi.org/10.1186/s13054-015-0820-1
Wang Q, Wang Y, West TM, Liu Y, Reddy GR, Barbagallo F, Xu B, Shi Q, Deng B, Wei W, Xiang YK (2021) Carvedilol induces biased beta1 adrenergic receptor-nitric oxide synthase 3-cyclic guanylyl monophosphate signalling to promote cardiac contractility. Cardiovasc Res 117:2237–2251. https://doi.org/10.1093/cvr/cvaa266
Woo AY, Xiao RP (2012) beta-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol Sin 33:335–341. https://doi.org/10.1038/aps.2011.201
Xiao H, Li H, Wang JJ, Zhang JS, Shen J, An XB, Zhang CC, Wu JM, Song Y, Wang XY, Yu HY, Deng XN, Li ZJ, Xu M, Lu ZZ, Du J, Gao W, Zhang AH, Feng Y, Zhang YY (2018) IL-18 cleavage triggers cardiac inflammation and fibrosis upon beta-adrenergic insult. Eur Heart J 39:60–69. https://doi.org/10.1093/eurheartj/ehx261
Yang P, Chen Z, Huang W, Zhang J, Zou L, Wang H (2023) Communications between macrophages and cardiomyocytes. Cell Commun Signal 21:206. https://doi.org/10.1186/s12964-023-01202-4
Zakyrjanova GF, Tsentsevitsky AN, Kuznetsova EA, Petrov AM (2021) Immune-related oxysterol modulates neuromuscular transmission via non-genomic liver X receptor-dependent mechanism. Free Radic Biol Med 174:121–134. https://doi.org/10.1016/j.freeradbiomed.2021.08.013
Zhai R, Snyder J, Montgomery S, Sato PY (2022) Double life: how GRK2 and beta-arrestin signaling participate in diseases. Cell Signal 94:110333. https://doi.org/10.1016/j.cellsig.2022.110333
Zhang ZS, Cheng HJ, Onishi K, Ohte N, Wannenburg T, Cheng CP (2005) Enhanced inhibition of L-type Ca2+ current by beta3-adrenergic stimulation in failing rat heart. J Pharmacol Exp Ther 315:1203–1211. https://doi.org/10.1124/jpet.105.089672
Zhang X, Zheng M, Kim KM (2020) GRK2-mediated receptor phosphorylation and Mdm2-mediated beta-arrestin2 ubiquitination drive clathrin-mediated endocytosis of G protein-coupled receptors. Biochem Biophys Res Commun 533:383–390. https://doi.org/10.1016/j.bbrc.2020.09.030
Michel LYM, Farah C, Balligand JL (2020) The beta3 adrenergic receptor in healthy and pathological cardiovascular tissues. Cells 9. https://doi.org/10.3390/cells9122584
Manna M, Niemela M, Tynkkynen J, Javanainen M, Kulig W, Muller DJ, Rog T, Vattulainen I (2016) Mechanism of allosteric regulation of beta(2)-adrenergic receptor by cholesterol. Elife 5. https://doi.org/10.7554/eLife.18432
Kawaguchi S, Okada M (2021) Cardiac metabolism in sepsis. Metabolites 11. https://doi.org/10.3390/metabo11120846
de Freitas FA, Levy D, Reichert CO, Cunha-Neto E, Kalil J, Bydlowski SP (2022) Effects of Oxysterols on Immune Cells and Related Diseases. Cells 11. https://doi.org/10.3390/cells11081251
Berthouze-Duquesnes M, Lucas A, Sauliere A, Sin YY, Laurent AC, Gales C, Baillie G, Lezoualc’h F (2013) Specific interactions between Epac1, beta-arrestin2 and PDE4D5 regulate beta-adrenergic receptor subtype differential effects on cardiac hypertrophic signaling. Cell Signal 25:970-980. https://doi.org/10.1016/j.cellsig.2012.12.007
Acknowledgements
We thank Dr. Nicole El-Darzi (Case Western Reserve University) for helpful comments on the manuscript and Dr. Andrey Zakharov (Kazan Federal University) for excellent technical assistance.
Funding
This work was supported by the Russian Science Foundation (grant no. 22-25-00396, https://rscf.ru/project/22-25-00396/).
Author information
Authors and Affiliations
Contributions
JGO supervised the study, prepared Figures 1–6, and performed experiments (calcium imaging, contraction recording); IRK, NAT, and DAT performed experiments (DAR-4M AM); ASA performed experiments (contraction recording) and prepared supplementary figure 1; AMP wrote the main manuscript text. AMP and JGO designed of the work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experimental protocol met the requirements of the EU Directive 2010/63/EU and was approved by the Local Ethical Committee of Kazan Medical University (Protocol #5 / May 27, 2014). The current study was conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Research does not involve human patients.
Consent to participate
Not applicable.
Consent for publication
All authors agree with the content of the manuscript, reviewed the manuscript, approved the final version. All authors consent to publication.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. All authors consent to publish the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 250 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Odnoshivkina, J.G., Averin, A.S., Khakimov, I.R. et al. The mechanism of 25-hydroxycholesterol-mediated suppression of atrial β1-adrenergic responses. Pflugers Arch - Eur J Physiol 476, 407–421 (2024). https://doi.org/10.1007/s00424-024-02913-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-024-02913-4