Abstract
Purpose
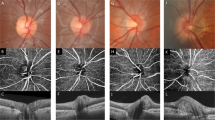
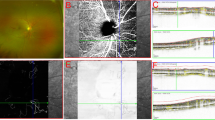
Peripapillary hyperreflective ovoid mass-like structures (PHOMS) represent an optical coherence tomography (OCT) finding that has been characterized in different forms of pseudopapilledema. The aim of this study was to investigate the prevalence of PHOMS in patients affected by acute LHON using structural OCT, and to provide a detailed description of these findings.
Methods
Patients with a clinical and molecularly confirmed diagnosis of acute LHON (visual loss having occurred less than 6 months) were enrolled from the neuro-ophthalmology clinic at San Raffaele Scientific Institute. Patients had a complete ophthalmologic evaluation, including imaging with structural OCT.
Results
Our analysis included 16 patients (21 eyes—8 males and 8 females) with acute LHON. Structural OCT exhibited PHOMS in 12 eyes from 9 patients with a prevalence rate of 57.1%. In a subsequent topographical assessment in the peripapillary area, the most common location of PHOMS was the temporal region (12 out of 12 eyes), while the nasal region was affected in 2 eyes (16.7%). Considering the 12 eyes with PHOMS, mean ± SD temporal peripapillary RNFL thickness was 87.5 ± 28.4 microns. The temporal peripapillary RNFL thickness was significantly lower in eyes without PHOMS (63.7 ± 32.2 microns; P = 0.40). At the 12-month follow-up visit, PHOMS disappeared in 10 out of 12 eyes.
Conclusions
Acute LHON eyes have PHOMS which are mainly confined to the temporal peripapillary sector. PHOMS may represent swelled retinal fibers that have herniated or are in stasis.


Similar content being viewed by others
References
Bassi ST, Mohana KP (2014) Optical coherence tomography in papilledema and pseudopapilledema with and without optic nerve head drusen. Indian J Ophthalmol 62(12):1146–1151
Pichi F, Romano S, Villani E et al (2014) Spectral-domain optical coherence tomography findings in pediatric tilted disc syndrome. Graefe’s Arch Clin Exp Ophthalmol 252(10):1661–1667
Malmqvist L, Bursztyn L, Costello F et al (2018) The optic disc drusen studies consortium recommendations for diagnosis of optic disc drusen using optical coherence tomography. J Neuro-Ophthalmology 38(3):299–307
Wang DD, Leong JCY, Gale J, Wells AP (2018) Multimodal imaging of buried optic nerve head drusen. Eye 32(6):1145–1146
Shinohara K, Moriyama M, Shimada N et al (2013) Analyses of shape of eyes and structure of optic nerves in eyes with tilted disc syndrome by swept-source optical coherence tomography and three-dimensional magnetic resonance imaging. Eye 27(11):1233–1241
Sibony PA, Kupersmith MJ (2016) Paton’s folds revisited: wrinkles, folds and creases in papilledema. Ophthalmology 123(6):1397–1399
Tso MO (1981) Pathology and pathogenesis of drusen of the optic nervehead. Ophthalmology 88(10):1066–1080
Malmqvist L, Bursztyn L, Costello F et al (2018) Peripapillary hyperreflective ovoid mass-like structures: is it optic disc drusen or not?: response. J Neuro-Ophthalmology 38(4):568–570
Carelli V, Ross-Cisneros FN, Sadun AA (2004) Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 23(1):53–89
Yu-Wai-Man P, Griffiths PG, Chinnery PF (2011) Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Prog Retin Eye Res 30(2):81–114
Carelli V, La Morgia C, Iommarini L et al (2007) Mitochondrial optic neuropathies: how two genomes may kill the same cell type? Biosci Rep 27(1–3):173–184
Newman NJ (2005) Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol 140(3):517–523
Sadun AA, Win PH, Ross-Cisneros FN et al (2000) Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc 98:223–232
Pan BX, Ross-Cisneros FN, Carelli V et al (2012) Mathematically modeling the involvement of axons in Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 53(12):7608–7617
Sadun AA, La Morgia C, Carelli V (2013) Mitochondrial optic neuropathies: our travels from bench to bedside and back again. Clin Exp Ophthalmol 41:702–712
Barboni P, Carbonelli M, Savini G et al (2010) Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology 117:623–627
Man PYW, Griffiths PG, Brown DT et al (2003) The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet 72:333–339
Hwang TJ, Karanjia R, Moraes-Filho MN et al (2017) Natural history of conversion of Leber’s hereditary optic neuropathy: a prospective case series. Ophthalmology 124:843–850
Barboni P, Savini G, Valentino ML et al (2005) Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology 112:127–131
Borrelli E, Barboni P, Battista M et al (2021) Peripapillary hyperreflective ovoid mass-like structures (PHOMS): OCTA may reveal new findings. Eye (Lond) 35:528–531
Wang D, Liu H-L, Du Y-Y et al (2021) Characterisation of thickness changes in the peripapillary retinal nerve fibre layer in patients with Leber’s hereditary optic neuropathy. Br J Ophthalmol 105:1166 LP – 1171
Balducci N, Savini G, Cascavilla ML et al (2016) Macular nerve fibre and ganglion cell layer changes in acute Leber’s hereditary optic neuropathy. Br J Ophthalmol 100:1232–1237
Borrelli E, Balasubramanian S, Triolo G et al (2018) Topographic macular microvascular changes and correlation with visual loss in chronic Leber hereditary optic neuropathy. Am J Ophthalmol 192:217–228
Borrelli E, Berni A, Cascavilla ML et al (2023) Visual outcomes and optical coherence tomography biomarkers of vision improvement in patients with Leber hereditary optic neuropathy treated with idebenone. Am J Ophthalmol 247:35–41
Carbonelli M, La Morgia C, Romagnoli M et al (2022) Capturing the pattern of transition from carrier to affected in Leber hereditary optic neuropathy. Am J Ophthalmol 241:71–79
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Turin and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Enrico Borrelli and Maria Lucia Cascavilla contributed equally to the work presented here and should therefore be regarded as equivalent authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borrelli, E., Cascavilla, M.L., Lari, G. et al. Peripapillary hyperreflective ovoid mass-like structures (PHOMS) in patients with acute Leber’s hereditary optic neuropathy. Graefes Arch Clin Exp Ophthalmol 262, 261–265 (2024). https://doi.org/10.1007/s00417-023-06205-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06205-y