Abstract
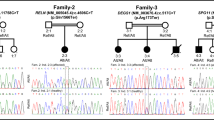
Sleep-related hypermotor epilepsy (SHE) is a focal epilepsy syndrome. The underlying pathophysiology is presumed to be closely related with disruption of GABAergic neurotransmission, which is mainly medicated by γ-aminobutyric acid type A receptor (GABAAR). Thus, it is reasonable to assume that rare GABAAR variants might contribute to the pathogenesis of SHE. To test this hypothesis, we performed next-generation sequencing in 58 SHE patients and analyzed the functional effects of the identified variants in both neuronal and non-neuronal cells using a combination of electrophysiology recordings, western blot, flow cytometry, and confocal microscopy. In our study, we detected three rare variants (NM_198904.2: c.269C > T, p.T90M; NM_198904.2: c.950C > A, p.T317N and NM_198903.2: c.649C > T, p.Q217X) in GABRG2 (MIM:137,164, encoding GABAAR γ2 subunit) in three unrelated patients. Two of the three rare variants were transmitted unaffected maternally (T90M) or unaffected paternally (Q217X), whereas the T317N variant arose de novo. The mother of proband carrying the T90M variant was unaffected and being mosaicism for this variant. Functional analysis showed that T90M and T317N variants decreased GABA-evoked current amplitudes by diverse mechanisms including impaired surface expression, endoplasmic reticulum retention, and channel gating defects. And Q217X variant reduced synaptic clustering and distribution of GABAAR. While a causal role of these variants cannot be established directly from these results, the functional assessment together with the genetic sequencing suggests that these rare GABRG2 variants may constitute genetic risk factors for SHE. Our study further expands the GABRG2 phenotypic spectrum and supports the view that GABAergic neurotransmission participates in the epileptogenesis of SHE.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Tinuper P, Bisulli F, Cross JH, Hesdorffer D, Kahane P, Nobili L, Provini F, Scheffer IE, Tassi L, Vignatelli L et al (2016) Definition and diagnostic criteria of sleep-related hypermotor epilepsy. Neurology 86:1834–1842. https://doi.org/10.1212/WNL.0000000000002666
Provini F, Plazzi G, Tinuper P, Vandi S, Lugaresi E, Montagna P (1999) Nocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive cases. Brain 122(Pt 6):1017–1031. https://doi.org/10.1093/brain/122.6.1017
Scheffer IE, Bhatia KP, Lopes-Cendes I, Fish DR, Marsden CD, Andermann F, Andermann E, Desbiens R, Cendes F, Manson JI (1994) Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet 343:515–517. https://doi.org/10.1016/s0140-6736(94)91463-x
Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer IE, Berkovic SF (1995) A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 11:201–203. https://doi.org/10.1038/ng1095-201
De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, Ballabio A, Wanke E, Casari G (2000) The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet 26:275–276. https://doi.org/10.1038/81566
Combi R, Ferini-Strambi L, Montruccoli A, Bianchi V, Malcovati M, Zucconi M, Dalprà L, Tenchini ML (2005) Two new putative susceptibility loci for ADNFLE. Brain Res Bull 67:257–263. https://doi.org/10.1016/j.brainresbull.2005.06.032
Becchetti A, Aracri P, Meneghini S, Brusco S, Amadeo A (2015) The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front Physiol 6:22. https://doi.org/10.3389/fphys.2015.00022
Licchetta L, Pippucci T, Baldassari S, Minardi R, Provini F, Mostacci B, Plazzi G, Tinuper P, Bisulli F (2020) Sleep-related hypermotor epilepsy (SHE): Contribution of known genes in 103 patients. Seizure 74:60–64. https://doi.org/10.1016/j.seizure.2019.11.009
Ishida S, Picard F, Rudolf G, Noé E, Achaz G, Thomas P, Genton P, Mundwiller E, Wolff M, Marescaux C et al (2013) Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat Genet 45:552–555. https://doi.org/10.1038/ng.2601
Ricos MG, Hodgson BL, Pippucci T, Saidin A, Ong YS, Heron SE, Licchetta L, Bisulli F, Bayly MA, Hughes J et al (2016) Mutations in the mammalian target of rapamycin pathway regulators NPRL2 and NPRL3 cause focal epilepsy. Ann Neurol 79:120–131. https://doi.org/10.1002/ana.24547
Korenke G-C, Eggert M, Thiele H, Nürnberg P, Sander T, Steinlein OK (2016) Nocturnal frontal lobe epilepsy caused by a mutation in the GATOR1 complex gene NPRL3. Epilepsia 57:e60–e63. https://doi.org/10.1111/epi.13307
Heron SE, Smith KR, Bahlo M, Nobili L, Kahana E, Licchetta L, Oliver KL, Mazarib A, Afawi Z, Korczyn A et al (2012) Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 44:1188–1190. https://doi.org/10.1038/ng.2440
Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J (2006) Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci USA 103:19152–19157. https://doi.org/10.1073/pnas.0608215103
Zhu G, Okada M, Yoshida S, Ueno S, Mori F, Takahara T, Saito R, Miura Y, Kishi A, Tomiyama M et al (2008) Rats harboring S284L Chrna4 mutation show attenuation of synaptic and extrasynaptic GABAergic transmission and exhibit the nocturnal frontal lobe epilepsy phenotype. J Neurosci 28:12465–12476. https://doi.org/10.1523/JNEUROSCI.2961-08.2008
Fukuyama K, Fukuzawa M, Shiroyama T, Okada M (2020) Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor. Br J Pharmacol 177:2143–2162. https://doi.org/10.1016/j.biopha.2020.110070
Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. https://doi.org/10.1038/nrn1625
Maljevic S, Møller RS, Reid CA, Pérez-Palma E, Lal D, May P, Lerche H (2019) Spectrum of GABAA receptor variants in epilepsy. Curr Opin Neurol 32:183–190. https://doi.org/10.1097/WCO.0000000000000657
Kang J-Q, Macdonald RL (2016) Molecular pathogenic basis for GABRG2 mutations associated with a spectrum of epilepsy syndromes, from generalized absence epilepsy to dravet syndrome. JAMA Neurol 73:1009–1016. https://doi.org/10.1001/jamaneurol.2016.0449
Jin P, Zhang J, Rowe-Teeter C, Yang J, Stuve LL, Fu GK (2004) Cloning and characterization of a GABAA receptor gamma2 subunit variant. J Biol Chem 279:1408–1414. https://doi.org/10.1074/jbc.M308656200
Schweizer C, Balsiger S, Bluethmann H, Mansuy IM, Fritschy J-M, Mohler H, Lüscher B (2003) The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci 24:442–450. https://doi.org/10.1016/s1044-7431(03)00202-1
Luscher B, Fuchs T, Kilpatrick CL (2011) GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70:385–409. https://doi.org/10.1016/j.neuron.2011.03.024
Huang X, Tian M, Hernandez CC, Hu N, Macdonald RL (2012) The GABRG2 nonsense mutation, Q40X, associated with Dravet syndrome activated NMD and generated a truncated subunit that was partially rescued by aminoglycoside-induced stop codon read-through. Neurobiol Dis 48:115–123. https://doi.org/10.1016/j.nbd.2012.06.013
Johnston AJ, Kang J-Q, Shen W, Pickrell WO, Cushion TD, Davies JS, Baer K, Mullins JGL, Hammond CL, Chung S-K et al (2014) A novel GABRG2 mutation, p. R136*, in a family with GEFS+ and extended phenotypes. Neurobiol Dis 64:131–141. https://doi.org/10.1016/j.nbd.2013.12.013
Macdonald RL, Kang J-Q (2012) mRNA surveillance and endoplasmic reticulum quality control processes alter biogenesis of mutant GABAA receptor subunits associated with genetic epilepsies. Epilepsia 53(Suppl 9):59–70. https://doi.org/10.1111/epi.12035
Warner TA, Shen W, Huang X, Liu Z, Macdonald RL, Kang J-Q (2016) Differential molecular and behavioural alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy. Hum Mol Genet 25:3192–3207. https://doi.org/10.1093/hmg/ddw168
Reid CA, Kim T, Phillips AM, Low J, Berkovic SF, Luscher B, Petrou S (2013) Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology 80:1003–1008. https://doi.org/10.1212/WNL.0b013e3182872867
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Licchetta L, Bisulli F, Vignatelli L, Zenesini C, Di Vito L, Mostacci B, Rinaldi C, Trippi I, Naldi I, Plazzi G et al (2017) Sleep-related hypermotor epilepsy: Long-term outcome in a large cohort. Neurology 88:70–77. https://doi.org/10.1212/WNL.0000000000003459
Licchetta L, Vignatelli L, Zenesini C, Mostacci B, Ferri L, Provini F, Tinuper P, Bisulli F (2019) Sleep-related hypermotor epilepsy: A prediction cohort study on sleep/awake patterns of seizures. Epilepsia 60:e115–e120. https://doi.org/10.1111/epi.16369
Todd E, Gurba KN, Botzolakis EJ, Stanic AK, Macdonald RL (2014) GABAA receptor biogenesis is impaired by the γ2 subunit febrile seizure-associated mutation, GABRG2(R177G). Neurobiol Dis 69:215–224. https://doi.org/10.1016/j.nbd.2014.05.013
Phillips HA, Marini C, Scheffer IE, Sutherland GR, Mulley JC, Berkovic SF (2000) A de novo mutation in sporadic nocturnal frontal lobe epilepsy. Ann Neurol 48:264–267
Baulac S, Ishida S, Marsan E, Miquel C, Biraben A, Nguyen DK, Nordli D, Cossette P, Nguyen S, Lambrecq V et al (2015) Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol 77:675–683. https://doi.org/10.1002/ana.24368
Stosser MB, Lindy AS, Butler E, Retterer K, Piccirillo-Stosser CM, Richard G, McKnight DA (2018) High frequency of mosaic pathogenic variants in genes causing epilepsy-related neurodevelopmental disorders. Genet Med 20:403–410. https://doi.org/10.1038/gim.2017.114
Spinner NB, Conlin LK (2014) Mosaicism and clinical genetics. Am J Med Genet C Semin Med Genet 166C:397–405. https://doi.org/10.1002/ajmg.c.31421
Cohen ASA, Wilson SL, Trinh J, Ye XC (2015) Detecting somatic mosaicism: considerations and clinical implications. Clin Genet 87:554–562. https://doi.org/10.1111/cge.12502
Iffland PH, Carson V, Bordey A, Crino PB (2019) GATORopathies: The role of amino acid regulatory gene mutations in epilepsy and cortical malformations. Epilepsia 60:2163–2173. https://doi.org/10.1111/epi.16370
Kang J-Q, Shen W, Macdonald RL (2013) Trafficking-deficient mutant GABRG2 subunit amount may modify epilepsy phenotype. Ann Neurol 74:547–559. https://doi.org/10.1002/ana.23947
Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J (2003) Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63:332–341. https://doi.org/10.1124/mol.63.2.332
Hernandez CC, Kong W, Hu N, Zhang Y, Shen W, Jackson L, Liu X, Jiang Y, Macdonald RL (2017) Altered channel conductance states and gating of GABA receptors by a pore mutation linked to Dravet syndrome. eNeuro. https://doi.org/10.1523/ENEURO.0251-16.2017
Shen D, Hernandez CC, Shen W, Hu N, Poduri A, Shiedley B, Rotenberg A, Datta AN, Leiz S, Patzer S et al (2017) De novo GABRG2 mutations associated with epileptic encephalopathies. Brain 140:49–67. https://doi.org/10.1093/brain/aww272
Feng Y, Tang B, Chen M, Yang L (2017) Whole-cell Currents Induced by Puff Application of GABA in Brain Slices. J Vis Exp 128:56387. https://doi.org/10.3791/56387
Bianchi MT, Macdonald RL (2002) Slow phases of GABA(A) receptor desensitization: structural determinants and possible relevance for synaptic function. J Physiol. https://doi.org/10.1113/jphysiol.2002.020255
Kang J-Q, Macdonald RL (2009) Making sense of nonsense GABA(A) receptor mutations associated with genetic epilepsies. Trends Mol Med 15:430–438. https://doi.org/10.1016/j.molmed.2009.07.003
Acknowledgements
We gratefully acknowledge Dr. Yang Ying for making critical comments for interpretation of the genetic variants identified. We thank Drs. Xiaoli Wang, Lang Jin, and Beibei Chen for technical assistance. We also thank the patients for their participation in this study.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No.81974204).
Author information
Authors and Affiliations
Contributions
YJ: Conceptualization, writing-original draft preparation, methodology, formal analysis, visualization. CS, HZ, and BF: Investigation, methodology, formal analysis, visualization. JZ, YL, and YM: Methodology, formal analysis, visualization. JH, SL: Conceptualization, writing-reviewing and editing, visualization, supervision. WJ: Conceptualization, writing-reviewing and editing, visualization, supervision, funding acquisition.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the Ethics Committee of Xijing Hospital and conducted in agreement with the relevant guidelines and regulations.
Consent to participate
Written informed consent was obtained from the patients and their parents/legal guardians.
Consent for publication
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Yl., Song, Cg., Zhou, Hm. et al. Rare variants in GABRG2 associated with sleep-related hypermotor epilepsy. J Neurol 269, 4939–4954 (2022). https://doi.org/10.1007/s00415-022-11137-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11137-4