Abstract
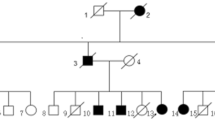
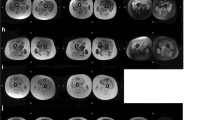
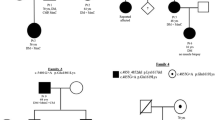
Autosomal dominant limb girdle muscular dystrophy D3 HNRNPDL-related is a rare dominant myopathy caused by mutations in HNRNPDL. Only three unrelated families have been described worldwide, a Brazilian and a Chinese carrying the mutation c.1132G>A p.(Asp378Asn), and one Uruguayan with the mutation c.1132G>C p. (Asp378His), both mutations occurring in the same codon. The present study enlarges the clinical, morphological and muscle MRI spectrum of AD-HNRNPDL-related myopathies demonstrating the significant particularities of the disease. We describe two new unrelated Argentinean families, carrying the previously reported c.1132G>C p.(Asp378His) HNRNPDL mutation. There was a wide phenotypic spectrum including oligo-symptomatic cases, pure limb girdle muscle involvement or distal lower limb muscle weakness. Scapular winging was the most common finding, observed in all patients. Muscle MRIs of the thigh, at different stages of the disease, showed particular involvement of adductor magnus and vastus besides a constant preservation of the rectus femoris and the adductor longus muscles, defining a novel MRI pattern. Muscle biopsy findings were characterized by the presence of numerous rimmed vacuoles, cytoplasmic bodies, and abundant autophagic material at the histochemistry and ultrastructural levels. HNRNPDL-related LGMD D3 results in a wide range of clinical phenotypes from the classic proximal form of LGMD to a more distal phenotype. Thigh MRI suggests a specific pattern. Codon 378 of HNRNPDL gene can be considered a mutation hotspot for HNRNPDL-related myopathy. Pathologically, the disease can be classified among the autophagic rimmed vacuolar myopathies as with the other multisystem proteinopathies.






Similar content being viewed by others
References
Straub V, Murphy A, Udd B, LGMD workshop study group (2018) 229th ENMC international workshop: Limb girdle muscular dystrophies—nomenclature and reformed classification Naarden, the Netherlands, 17–19 March 2017. Neuromuscul Disord 28:702–710. https://doi.org/10.1016/j.nmd.2018.05.007
Starling A, Kok F, Passos-Bueno MR, Vainzof M, Zatz M (2004) A new form of autosomal dominant limb-girdle muscular dystrophy (LGMD1G) with progressive fingers and toes flexion limitation maps to chromosome 4p21. Eur J Hum Genet. 12:1033–1040
Vieira NM, Naslavsky MS, Licinio L, Kok F, Schlesinger D, Vainzof M, Sanchez N, Kitajima JP, Gal L, Cavacana N, Serafini PR, Chuartzman S, Vasquez C, Mimbacas A, Nigro V, Pavanello RC, Schuldiner M, Kunkel LM, Zatz M (2014) A defect in the RNA-processing protein HNRPDL causes limb-girdle muscular dystrophy 1G (LGMD1G). Hum Mol Genet 23:4103–4110. https://doi.org/10.1093/hmg/ddu127
Sun Y, Chen H, Lu Y, Duo J, Lei L, OuYang Y, Hao Y, Da Y, Shen X-M (2019) Limb girdle muscular dystrophy D3 HNRNPDL related in a Chinese family with distal muscle weakness caused by a mutation in the prion-like domain. J Neurol 266:498–506. https://doi.org/10.1007/s00415-018-9165-4
Li RZ, Hou J, Wei Y, Luo X, Ye Y, Zhang Y (2019) hnRNPDL extensively regulates transcription and alternative splicing. Gene 687:125–134. https://doi.org/10.1016/j.gene.2018.11.026
Kawamura H, Tomozoe Y, Akagi T, Kamei D, Ochiai M, Yamada M (2002) Identification of the nucleocytoplasmic shuttling sequence of heterogeneous nuclear ribonucleoprotein D-like protein JKTBP and its interaction with mRNA. J Biol Chem 277:2732–2739
Kamei D, Yamada M (2002) Interactions of heterogeneous nuclear ribonucleoprotein D-like protein JKTBP and its domains with high-affinity binding sites. Gene 298:49–57
Geuens T, Bouhy D, Timmerman V (2016) The hnRNP family: insights into their role in health and disease. Hum Genet 135:851–867. https://doi.org/10.1007/s00439-016-1683-5
Izumi R, Warita H, Niihori T, Takahashi T, Tateyama M, Suzuki N, Nishiyama A, Shirota M, Funayama R, Nakayama K, Mitsuhashi S, Nishino I, Aoki Y, Aoki M (2015) Isolated inclusion body myopathy caused by a multisystem proteinopathy-linked hnRNPA1 mutation. Neurol Genet 1:e23. https://doi.org/10.1212/NXG.0000000000000023
Kim HJ, Kim NC, Wang YD, Scarborough EA, Moore J, Diaz Z, MacLea KS, Freibaum B, Li S, Molliex A, Kanagaraj AP, Carter R, Boylan KB, Wojtas AM, Rademakers R, Pinkus JL, Greenberg SA, Trojanowski JQ, Traynor BJ, Smith BN, Topp S, Gkazi AS, Miller J, Shaw CE, Kottlors M, Kirschner J, Pestronk A, Li YR, Ford AF, Gitler AD, Benatar M, King OD, Kimonis VE, Ross ED, Weihl CC, Shorter J, Taylor JP (2013) Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495:467–473. https://doi.org/1038/nature11922
Mercuri E, Cini C, Pichiecchio A, Allsop J, Counsell S, Zolkipli Z, Messina S, Kinali M, Brown SC, Jimenez C, Brockington M, Yuva Y, Sewry CA, Muntoni F (2003) Muscle magnetic resonance imaging in patients with congenital muscular dystrophy and Ullrich phenotype. Neuromuscular Disord 13:554–558
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F (2007) Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging 25:433–440
Kottlors M, Moske-Eick O, Huebner A, Krause S, Mueller K, Kress W, Schwarzwald R, Bornemann A, Haug V, Heitzer M, Kirschner J (2010) Late-onset autosomal dominant limb girdle muscular dystrophy and Paget's disease of bone unlinked to the VCP gene locus. J Neurol Sci 291:79–85. https://doi.org/10.1016/j.jns.2009.12.008
Huovinen S, Penttilä S, Somervuo P, Keto J, Auvinen P, Vihola A, Huovinen S, Pelin K, Raheem O, Salenius J, Suominen T, Hackman P, Udd B (2015) Differential isoform expression and selective muscle involvement in muscular dystrophies. Am J Pathol 185:2833–2842. https://doi.org/10.1016/j.ajpath.2015.06.018
Lee Y, Jonson PH, Sarparanta J, Palmio J, Sarkar M, Vihola A, Evilä A, Suominen T, Penttilä S, Savarese M, Johari M, Minot MC, Hilton-Jones D, Maddison P, Chinnery P, Reimann J, Kornblum C, Kraya T, Zierz S, Sue C, Goebel H, Azfer A, Ralston SH, Hackman P, Bucelli RC, Taylor JP, Weihl CC, Udd B (2018) TIA1 variant drives myodegeneration in multisystem proteinopathy with SQSTM1 mutations. J Clin Invest 128:1164–1177. https://doi.org/10.1172/JCI97103
Acknowledgements
This work has been financially supported by Association Françaisecontre les Myopathies (AFM-Telethon), Association Institut de Myologie (AIM), Assistance Publique-Hôpitaux de Paris (AP-HP), Institut National de la Santé et de la RechercheMédicale (INSERM), Sorbonne Université, Centre National de la RechercheScientifique (CNRS), University of Strasbourg, the France Génomique National infrastructure funded as part of the Investissementsd’ Avenir program managed by the AgenceNationale pour la Recherche (ANR-10-INBS-09) and by Fondation Maladies Rares within the frame of the “Myocapture” sequencing project, the Fondation pour la RechercheMédicale.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors have reviewed the manuscript. Authors have no conflict of interest to declare.
Ethical standards
The study was conducted after receiving written informed consent from patients in accordance to the declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Berardo, A., Lornage, X., Johari, M. et al. HNRNPDL-related muscular dystrophy: expanding the clinical, morphological and MRI phenotypes. J Neurol 266, 2524–2534 (2019). https://doi.org/10.1007/s00415-019-09437-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09437-3