Abstract
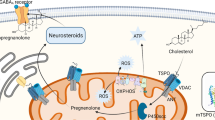
There is need for novel fast acting treatment options in affective disorders. 3α-reduced neurosteroids such as allopregnanolone are powerful positive allosteric modulators of GABAA receptors and target also extrasynaptic receptors. Their synthesis is mediated by the translocator protein 18 kDa (TSPO). TSPO ligands not only promote endogenous neurosteroidogenesis, but also exert a broad spectrum of functions involving modulation of mitochondrial activity and acting as anti-inflammatory and neuroregenerative agents. Besides affective symptoms, in depression cognitive impairment can be frequently observed, which may be ameliorated through targeting of extrasynaptic GABAA receptors either via TSPO ligands or exogenously administered 3α-reduced neurosteroids. Interestingly, recent findings indicate an enhanced activation of the complement system, e.g., enhanced expression of C1q, both in depression and dementia. It is of note that benzodiazepines have been shown to reduce long-term potentiation and to cause cognitive decline. Intriguingly, TSPO may be crucial in mediating the effects of benzodiazepines on synaptic pruning. Here, we discuss how benzodiazepines and TSPO may interfere with synaptic pruning. Moreover, we highlight recent developments of TSPO ligands and 3α-reduced neurosteroids as therapeutic agents. Etifoxine is the only clinically available TSPO ligand so far and has been studied in anxiety disorders. Regarding 3α-reduced neurosteroids, brexanolone, an intravenous formulation of allopregnanolone, has been approved for the treatment of postpartum depression and zuranolone, an orally available 3α-reduced neurosteroid, is currently being studied in major depressive disorder and postpartum depression. As such, 3α-reduced neurosteroids and TSPO ligands may constitute promising treatment approaches for affective disorders.




Similar content being viewed by others
Data availability
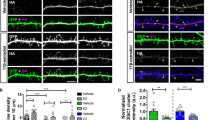
This review does not contain original data with the exception of Figure 4, which was generated in the laboratory of Gerhard Rammes, Experimental Neuropharmacology, Department of Anesthesiology, Technical University Munich.
References
Cheng Q, Huang J, Xu L, Li Y, Li H, Shen Y et al (2020) Analysis of time-course, dose-effect, and influencing factors of antidepressants in the treatment of acute adult patients with major depression. Int J Psychopharmacol 23:76–87. https://doi.org/10.1093/ijnp/pyz062
Kritzer MD, Mischel NA, Young JR, Lai CS, Masand PS, Szabo ST et al (2022) Ketamine for treatment of mood disorders and suicidality: a narrative review of recent progress. Ann Clin Psychiatry 34:33–43. https://doi.org/10.12788/acp.0048
Saiz-Vazquez O, Gracia-Garcia P, Ubillos-Landa S, Puente-Martinez A, Casado-Yusta S, Olaya B, Santabarbara J (2021) Depression as a risk factor for Alzheimer’s disease: a systematic review of longitudinal meta-analyses. J Clin Med. https://doi.org/10.3390/jcm10091809
Pariente A, de Gage SB, Moore N, Bégaud B (2016) The benzodiazepine–dementia disorders link: current state of knowledge. CNS Drugs 30:1–7. https://doi.org/10.1007/s40263-015-0305-4
Barker MJ, Greenwood KM, Jackson M, Crowe SF (2004) Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs 18:37–48. https://doi.org/10.2165/00023210-200418010-00004
Zhang Y, Zhou X, Meranus DH, Wang L, Kukull WA (2016) Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis Assoc Disord 30:113–117. https://doi.org/10.1097/WAD.0000000000000099
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR et al (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27:402–409. https://doi.org/10.1016/j.tips.2006.06.005
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N et al (2010) Translocator protein (18 kDa) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9:971–988. https://doi.org/10.1038/nrd3295
Rupprecht R, Rupprecht C, Rammes G (2021) Neuroinflammation and psychiatric disorders: relevance of C1q, translocator protein (18kDa) (TSPO), and neurosteroids. World J Biol Psychiatry 10:1–7. https://doi.org/10.1080/15622975.2021.1961503
Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V (2015) Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci USA 112:7261–7266. https://doi.org/10.1073/pnas.1502670112
Selvaraj V, Stocco DM, Tu LN (2015) Minireview: translocator protein (TSPO) and steroidogenesis: a reappraisal. Mol Endocrinol 2:490–501. https://doi.org/10.1210/me.2015-1033
Pini S, Martini C, Abelli M, Muti M, Gesi C, Montali M, Chelli B, Lucacchini A, Cassano GB (2005) Peripheral-type benzodiazepine receptor binding sites in platelets of patients with panic disorder associated to separation anxiety symptoms. Psychopharmacology 181:407–411. https://doi.org/10.1007/s00213-005-2247-x
Abelli M, Chelli B, Costa B, Lari L, Cardini A, Gesi C, Muti M, Lucacchini A, Martini C, Cassano GB et al (2010) Reductions in platelet 18-kDa translocator protein density are associated with adult separation anxiety in patients with bipolar disorder. Neuropsychobiology 62:98–103. https://doi.org/10.1159/000315440
Sarubin N, Baghai TC, Lima-Ojeda JM, Melchner D, Hallof-Buestrich H, Wolf L, Hilbert S, Milenkovic VM, Wetzel CH, Rupprecht R et al (2016) Translocator protein (TSPO) expression in platelets of depressed patients decreases during antidepressant therapy. Pharmacopsychiatry 49:204–209. https://doi.org/10.1055/s-0042-107795
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S et al (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–265. https://doi.org/10.1001/jamapsychiatry.2014.2427
Attwells S, Setiawan E, Wilson AA, Rusjan PM, Mizrahi R, Miler L, Xu C, Richter MA, Kahn A, Kish SJ et al (2017) Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry 74:833–840. https://doi.org/10.1001/jamapsychiatry.2017.1567
Attwells S, Setiawan E, Rusjan OM, Xu C, Hutton C, Rafiei D, Varughese B, Kahn A, Kish SJ, Vasdev N (2020) Translocator protein distribution volume predicts reductions of symptoms during open-label trial of celecoxib in major depressive disorder. Biol Psychiatry 88:649–656. https://doi.org/10.1016/j.biopsych.2020.03.007
Tournier BB, Tsartsalis S, Ceyzeriat K, Garibotto V, Millet P (2020) In vivo TSPO signal and neuroinflammation in Alzheimer´s disease. Cells 9:1941. https://doi.org/10.3390/cells9091941
Li H, Sagar AP, Keri S (2018) Microglial markers in the frontal cortex are related to cognitive dysfunctions in major depressive disorder. J Affect Disord 241:305–310. https://doi.org/10.1016/j.jad.2018.08.021
Notter T, Schalbetter SM, Clifton NE, Mattei D, Richetto J, Thomas K et al (2021) Neuronal activity increases translocator protein (TSPO) levels. Mol Psychiatry 26:2025–2037. https://doi.org/10.1038/s41380-020-0745-1
Costa B, Pini S, Martini C, Abelli M, Gabelloni P, Landi S et al (2009) Ala147Thr substitution in translocator protein is associated with adult separation anxiety in patients with depression. Psychiatr Genet 19:110–111. https://doi.org/10.1097/YPG.0b013e32832080f6
Colasanti A, Owen DR, Grozeva D, Rabiner EA, Matthews PM, Craddock N et al (2013) Bipolar disorder is associated with the rs6971 polymorphism in the gene encoding 18 kDa translocator protein (TSPO). Psychoneuroendocrinology 38:2826–2829. https://doi.org/10.1016/j.psyneuen.2013.07.007
Prossin AR, Chandler M, Ryan KA, Saunders F, Kamali M, Papadopoulos V et al (2018) Functional TSPO polymorphism predicts variance in the diurnal cortisol rhythm in bipolar disorder. Psychoneuroendocrinology 89:194–202. https://doi.org/10.1016/j.psyneuen.2018.01.013
Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschiy JM et al (1999) Benzodiazepine actions mediated by specific gamma-aminobutyric acic(A) receptor subtypes. Nature 401:796–800. https://doi.org/10.1038/44579
Chen ZW, Bracamontes JR, Budelier MM, Germann AL, Shin DJ, Kathiresan K et al (2019) Multiple functional neurosteroid binding sites on GABAA receptors. PLoS Biol 17:e3000157. https://doi.org/10.1371/journal.pbio.3000157
Locci A, Pinna G (2017) Neurosteroid biosynthesis down-regulation and changes in GABA(A) receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol 174:3226–3241. https://doi.org/10.1111/bph.13843
Paul SM, Pinna G, Guidotti A (2020) Allopregnanolone: from molecular pathophysiology to therapeutics: a historical perspective. Neurobiol Stress 14:110215. https://doi.org/10.1016/j.ynstr.2020.100215
Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, Kazdoba TM, Mogdil A, Davies PA, Moss SJ et al (2020) Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABA(A) receptor positive allosteric modulator. Neuropharmacology 181:10833. https://doi.org/10.1016/j.neuropharm.2020.108333
Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S (2013) Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev 37:109–122. https://doi.org/10.1016/j.neubiorev.2012.10.005
Rupprecht R (2003) Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 28:139–168. https://doi.org/10.1016/s0306-4530(02)00064-1
Rupprecht R, Wetzel CH, Dorostkar M, Herms J, Albert NL, Schwarzbach J, Schumacher M, Neumann ID (2022) Translocator protein (18kDa) TSPO: a new diagnostic or therapeutic target for stress-related disorders? Mol Psychiatry 27:2918–2926. https://doi.org/10.1038/s41380-022-01561-3
Griffin LD, Mellon SH (1999) Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA 96:13512–13517. https://doi.org/10.1073/pnas.96.23.13512
Schüle C, Romeo E, Uzunov DP, Eser D, di Michele F, Baghai TC et al (2006) Influence of mirtazapine on plasma concentrations of neuroactive steroids in major depression and on 3alpha-hydoxysteroid oxidoreductase activity. Mol Psychiatry 11:261–272. https://doi.org/10.1038/sj.mp.4001782
Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R (1998) Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry 155:910–913. https://doi.org/10.1176/ajp.155.7.910
Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E et al (1998) Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl Acad Sci USA 95:3239–3244. https://doi.org/10.1073/pnas.95.6.3239
Ströhle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewski G, Holsboer F, Rupprecht R (2003) Induced panic attacks shift GABA(A) receptor modulatory steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry 60:161–168. https://doi.org/10.1001/archpsyc.60.2.161
Girdler SS, Klatzkin R (2007) Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther 116:125–139. https://doi.org/10.1016/j.pharmthera.2007.05.006
Hellgren C, Åkerud H, Skalkidou A, Bäckström T, Sundström-Poromaa I (2014) Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology 69:147–153. https://doi.org/10.1159/000358838
Maguire J, Mody I (2008) GABA(A)R plasticity during pregnancy: relevance to post-partum depression. Neuron 59:207–213. https://doi.org/10.1016/j.neuron.2008.06.019
Brickley SG, Mody I (2012) Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73:23–34. https://doi.org/10.1016/j.neuron.2011.12.012
Pinna G, Almeida FB, Davis JM (2022) Allopregnanolone in postpartum depression. Front Glob Womens Health 3:823616. https://doi.org/10.3389/fgwh.2022.823616
Deligiannidis KM, Kroll-Desrosiers AR, Tan Y, Dubuke ML, Shaffer SA (2020) Longitudinal proneuroactive and neuroactive steroid profiles in medication-free women with, without and at-risk for perinatal depression: a liquid chromatography-tandem mass spectrometry analysis. Psychoneuroendocrinology 121:104827. https://doi.org/10.1016/j.psyneuen.2020.104827
Timby E, Bäckström T, Nyberg S, Stenlund H, Wihlbäck AN, Bixo M (2016) Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls-a pilot study. Psychopharmacology 233:2109–2117. https://doi.org/10.1007/s00213-016-4258-1
Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F et al (2009) Translocator protein (18 kDa) as target for anxiolytics without benzodiazepine-like side effects. Science 325:490–493. https://doi.org/10.1126/science.1175055
Barron AM, Higuchi M, Hattori S, Kito S, Suhara T, Ji B (2021) Regulation of anxiety and depression by mitochondrial translocator protein-mediated steroidogenesis: the role of neurons. Mol Neurobiol 58:550–563. https://doi.org/10.1007/s12035-020-02136-5
Ren P, Ma L, Wang JY, Guo H, Sun L, Gao ML, Liu YZ, Ma YQ, Li FY, Guo WZ (2020) Anxiolytic and anti-depressive like effects of translocator protein (18 kDa) ligand YL-IPAo8 in a rat model of postpartum depression. Neurochem Res 45:1746–1757. https://doi.org/10.1007/s11064-020-03036-9
Nozaki K, Ito H, Ohgidani M, Yamawaki Y, Sahin EH, Kitajima T, Katsumata S, Yamawaki S, Kato TA, Aizawa H (2020) Antidepressant effect of the translocator protein antagonist ONO-2952 on mouse behaviors under chronic social defeat stress. Neuropharmacology 162:107835. https://doi.org/10.1016/j.neuropharm.2019.107835
Mattei C, Taly A, Soualah Z, Sauleis O, Herrison D, Guerineau NC, Verleye M, Legros C (2019) Involvement of the GABA(A) receptor α subunit in the mode of action of etifoxine. Pharmacol Res 145:104250. https://doi.org/10.1016/j.phrs.2019.04.034
Nguyen N, Fakra E, Pradel V, Jouve E, Alquier C, Le Guern ME, Micallef J, Blin O (2006) Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: a double-blind controlled study in general practice. Hum Psychopharmacol 21:139–149. https://doi.org/10.1016/j.neuropharm.2019.107835
Vincente B, Saldicia S, Hormazabal N, Bustos C, Rubi P (2020) Etifoxine is non-inferior than clonazepam for the reduction of anxiety disorders: a randomized, double blind, non-inferiority trial. Psychopharmacology 237:3357–3367. https://doi.org/10.1007/s00213-020-05617-6
Witkin JM, Lippa A, Smith JL, Jin X, Ping X, Biggerstaff A et al (2022) The imidazodiazepine, KRM-II-81: an example of a newly emerging generations of GABAkines for neurological and psychiatric disorders. Phamacol Biochem Behav 213:173321. https://doi.org/10.1016/j.pbb.2021.173321
Kriegeskorte N, Mur M, Bandettini PA (2008) Representational similarity analysis-connecting the branches of systems neuroscience. Front Syst Neurosci 2:4. https://doi.org/10.3389/neuro.06.004.2008
Levine SM, Pfaller M, Reichenberger J, Shiban Y, Mühlberger A, Rupprecht R, Schwarzbach JV (2018) Relating experimentally-induced fear to pre-existing phobic fear in the human brain. Soc Cogn Affect Neurosci 13:64–172. https://doi.org/10.1093/scan/nsx147
Thornton MA, Weaverdyck ME, Mildner JN, Tamir DI (2019) People represent their own mental states more distinctly than those of others. Nat Commun 10:1–9. https://doi.org/10.1038/s41467-019-10083-6
Kemp C, Tenenbaum JB (2008) The discovery of structural form. Proc Natl Acad Sci USA 105:10687–10692. https://doi.org/10.1073/pnas.0802631105
Levine SM, Wackerle A, Rupprecht R, Schwarzbach JV (2018) The neural representation of an individualized relational affective space. Neuropsychologia 120:35–42. https://doi.org/10.1016/j.neuropsychologia.2018.10.008
Levine SM, Schwarzbach JV (2021) Individualizing representational similarity analysis. Front Psychiatry 12:729457. https://doi.org/10.3389/fpsyt.2021.729457
Hong S, Dissing-Olesen L, Stevens B (2016) New insights on the role of microglia in synaptic pruning in health and disease. Curr Opin Neurobiol 36:128–134. https://doi.org/10.1016/j.conb.2015.12.004
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. https://doi.org/10.1016/j.cell.2007.10.036
Scott-Hewitt N, Perrucci F, Morini R, Erreni M, Mahoney M, Witkowska A, Carey A, Faggiani E, Schuetz LT, Mason S et al (2020) Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J 9:e105380. https://doi.org/10.15252/embj.2020105380
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson AC et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400. https://doi.org/10.1038/nature12776
Lee JH, Kim JY, Noh S, Lee H, Lee SY, Mun JY, Park H, Chung WS (2021) Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590:612–617. https://doi.org/10.1038/s41586-020-03060-3
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, RamakrishnanS MKM, Shi Q, Rosenthal A, Barres BA, Lemere CA, Selkoe DJ, Stevens B (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712–716. https://doi.org/10.1126/science.aad8373
Rupprecht C, Rupprecht R, Rammes G (2021) C1q, a small molecule with high impact on brain development: putative role for aging processes and the occurrence of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 271:809–812. https://doi.org/10.1007/s00406-021-01273-9
Roman C, Egert L, Di Benedetto B (2020) Astrocytic-neuronal crosstalk gets jammed: alternative perspectives on the onset of neuropsychiatric disorders. Eur J Neurosci 54:5717–5729. https://doi.org/10.1111/ejn.14900
Benoit E, Tenner AJ (2011) Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J Neurosci 31:3459–3469. https://doi.org/10.1523/JNEUROSCI.3932-10.2011
Rupprecht C, Sarker RSJ, Rammes G (2022) Morphological representation of C1q in the aging central nervous system. Pharmacopsychiatry 55:203–210. https://doi.org/10.1055/a-1704-8260
Tooyama I, Sato H, Yasuhara O, Kimura H, Konishi Y, Shen Y, Walker DG, Beach TG, Sue LI, Rogers J (2001) Correlation of the expression level of C1qmRNA and the number of C1q-positive plaques in the Alzheimer disease temporal cortex. Analysis of C1q mRNA and its protein using adjacent or nearby sections. Dement Geriatr Cogn Disord 12:237–242. https://doi.org/10.1159/000051265
Yang J, Li R, Shi Y, Jiang S, Liu J (2020) Is serum complement C1q related to major depressive disorder? Indian J Psychiatry 62:659–663. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_394_19
Yao Q, Li J (2020) Increased serum levels of complement C1q in major depressive disorder. J Psychosom Res 133:110105. https://doi.org/10.1016/j.jpsychores.2020.110105
Fairley LH, Sahara N, Aoki I, Ji B, Suhara T, Higuchi M, Barron AM (2021) Neuroprotective effect of mitochondrial translocator protein ligand in a mouse model of tauopathy. J Neuroinflammation 18:76. https://doi.org/10.1186/s12974-021-02122-1
van der Ende EL, Heller C, Sogorb-Esteve A, Swift IJ, McFall D, Peakman G, Bouzigues A, Poos JM, Jiskoot LC, Panman JL et al (2022) Elevated CSF and plasma complement proteins in genetic frontotemporal dementia: results from the GENFI study. J Neuroinflammation 19:217. https://doi.org/10.1186/s12974-022-02573-0
van der Ende EL, Bron EE, Poos JM, Jiskoot LC, Panman JL, Papma JM, Meeter LH, Dopper EGP, Wilke C, Synofzik M et al (2022) A data-driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain 145:1805–1817. https://doi.org/10.1093/brain/awab382
Brosseron F, Maass A, Kleineidam L, Ravichandran KA, González PG, McManus RM, Ising C, Santarelli F, Kolbe CC, Häsler LM et al (2022) Soluble TAM receptors sAXL and sTyro3 predict structural and functional protection in Alzheimer’s disease. Neuron 110:1009–1022. https://doi.org/10.1016/j.neuron.2021.12.016
Shi Y, Cui M, Ochs K, Strübing FL, Briel N, Eckenweber F et al (2022) Long-term diazepam treatment enhances microglial spine engulfment and impairs cognitive performance via the mitochondrial 18 kDa translocator protein (TSPO). Nat Neurosci 25:317–329. https://doi.org/10.1038/s41593-022-01013-9
Barker M, Greenwood KM, Jackson M, Crowe SF (2004) Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Arch Clin Neuropsychol 19:437–454. https://doi.org/10.1016/S0887-6177(03)00096-9
Zhang Y, Zhou X, Meranus DH, Wang L, Kukull WA (2016) Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis Assoc Disord 30:113–117. https://doi.org/10.1097/WAD.0000000000000099
de Gage SB, Moride Y, Ducruet T, Kurth T, Verdoux H, Tournier M et al (2014) Benzodiazepine use and risk of Alzheimer’s disease: case-control study. Br Med J 349:g5205–g5205. https://doi.org/10.1136/bmj.g5205
Biétry FA, Pfeil AM, Reich O, Schwenkglenks M, Meier CR (2017) Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs 31:245–251. https://doi.org/10.1007/s40263-016-0404-x
Gray SL, Dublin S, Yu O, Walker R, Anderson M, Hubbard RA et al (2016) Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. Br Med J 352:i90. https://doi.org/10.1136/bmj.i90
Puig-Bosch X, Bieletzki S, Zeilhofer HU, Rudolph U, Antkowiak B, Rammes G (2022) Midazolam at low nanomolar concentrations affects long-term potentiation and synaptic transmission predominantly via the α1-γ-aminobutyric acid type a receptor subunit in mice. Anesthesiology 136:954–969. https://doi.org/10.1097/ALN.0000000000004202
Powell JG, Garland S, Preston K, Piszczatoski C (2020) Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother Actions 54:157–163. https://doi.org/10.1177/1060028019873320
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, Meltzer-Brody S (2017) Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390:480–489. https://doi.org/10.1016/S0140-6736(17)31264-3
Meltzer-Brody S, Calquhoun H, Riesenberg R, Epperson CN, Deligiannidis K, Rubinow DR, Li H, Sankah AS, Glemson C, Schacterle A, Jonas J et al (2018) Brexanolone injection in post-partum depression: two multicentre, double blind, randomised, placebo-controlled, phase-3 trails. Lancet 392:1058–1070. https://doi.org/10.1016/S0140-6736(18)31551-4
Dichtel LE, Nyer M, Dording C, Fisher LB, Cusin C, Shapero BG, Pedrelli P, Kimball AS, Rao EM, Mischoulon D, Fava M, Miller KK (2020) Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. https://doi.org/10.4088/JCP.19m12887
Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, Li H, Lasser R, Zorumski CF, Rubinow DR, Paul SM, Jonas J, Doherty JJ, Kanes SJ (2019) Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med 381:903–911. https://doi.org/10.1056/NEJMoa1815981
Suthoff E, Kosinski M, Arnaud A, Hodgkins P, Gunduz-Bruce H, Lasser R, Silber C, Sankoh AJ, Li H, Werneburg B, Jonas J, Doherty J, Kanes SJ, Bonthapally V (2022) Patient-reported health-related quality of life from a randomized, placebo-controlled phase 2 trial of zuranolone in adults with major depressive disorder. J Affect Disord 308:19–26. https://doi.org/10.1016/j.jad.2022.03.068
Arnaud A, Suthoff E, Stenson K, Werneburg B, Hodgkins P, Bonthapally V, Jonas J, Meyer K, O’Day K (2021) Number needed to treat and number needed to harm analysis of the zuranolone phase 2 clinical trial results in major depressive disorder. J Affect Disord 285:112–119. https://doi.org/10.1016/j.jad.2021.02.027
Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, Sankoh AJ, Silber C, Campbell AD, Werneburg B, Kanes SJ, Lasser R (2021) Effect of zuranolone vs placebo in postpartum depression: a randomized clinical trial. JAMA Psychiatry 78:951–959. https://doi.org/10.1001/jamapsychiatry.2021.1559
Bullock A, Gunduz-Bruce H, Zammit GK, Qin M, Li H, Sankoh AJ, Silber C, Kanes SJ, Jonas J, Doherty J (2022) A phase 1 double-blind, placebo-controlled study of zuranolone (SAGE-217) in a phase advance model of insomnia in healthy adults. Hum Psychopharmacol 37:e2806. https://doi.org/10.1002/hup.2806
Schüle C, Nothdurfter C, Rupprecht R (2014) The role of allopregnanolone in depression and anxiety. Prog Neurobiol 113:79–87. https://doi.org/10.1016/j.pneurobio.2013.09.003
Rammes G, Hasenjager A, Sroka-Saidi K, Deussing JM, Parsons CG (2011) Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of beta-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology 60:982–990. https://doi.org/10.1016/j.neuropharm.2011.01.051
Rammes G, Gravius A, Ruitenberg M, Wegener N, Chambon C, Sroka-Saidi K, Jeggo R et al (2015) MRZ-99030–A novel modulator of Aβ aggregation: II - Reversal of Aβ oligomer-induced deficits in long-term potentiation (LTP) and cognitive performance in rats and mice. Neuropharmacology 92:170–182. https://doi.org/10.1016/j.neuropharm.2014.12.037
Acknowledgements
This work has been supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) (DFG), project number 422179811 to RR, CN and JS. Part of this work is part of the thesis of Christian Rupprecht at Faculty of Medicine, Technical University Munich, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RR has received consultancy honoraria from SAGE/Biogen and GABA Therapeutics.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rupprecht, R., Pradhan, A.K., Kufner, M. et al. Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options. Eur Arch Psychiatry Clin Neurosci 273, 1477–1487 (2023). https://doi.org/10.1007/s00406-022-01532-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01532-3