Abstract
Purpose
First-generation bone bridges (BBs) have demonstrated favorable safety and audiological benefits in patients with conductive hearing loss. However, studies on the effects of second-generation BBs are limited, especially among children. In this study, we aimed to explore the surgical and audiological effects of second-generation BBs in patients with bilateral congenital microtia.
Methods
This single-center prospective study included nine Mandarin-speaking patients with bilateral microtia. All the patients underwent BCI Generation 602 (BCI602; MED-EL, Innsbruck, Austria) implant surgery between September 2021 and June 2023. Audiological and sound localization tests were performed under unaided and BB-aided conditions.
Results
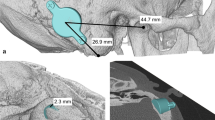
The transmastoid and retrosigmoid sinus approaches were implemented in three and six patients, respectively. No patient underwent preoperative planning, lifts were unnecessary, and no sigmoid sinus or dural compression occurred. The mean function gain at 0.5–4.0 kHz was 28.06 ± 4.55-dB HL. The word recognition scores improved significantly in quiet under the BB aided condition. Signal-to-noise ratio reduction by 10.56 ± 2.30 dB improved the speech reception threshold in noise. Patients fitted with a unilateral BB demonstrated inferior sound source localization after the initial activation.
Conclusions
Second-generation BBs are safe and effective for patients with bilateral congenital microtia and may be suitable for children with mastoid hypoplasia without preoperative three-dimensional reconstruction.





Similar content being viewed by others
Data, materials and/or code availability
The data are available from the corresponding author on reasonable request.
References
Luquetti DV, Heike CL, Hing AV et al (2012) Microtia: epidemiology and genetics. Am J Med Genet 158A:124–139. https://doi.org/10.1002/ajmg.a.34352
Shonka DC, Livingston WJ, Kesser BW (2008) The Jahrsdoerfer grading scale in surgery to repair congenital aural atresia. Arch Otolaryngol Head Neck Surg 134:873. https://doi.org/10.1001/archotol.134.8.873
Lo JFW, Tsang WSS, Yu JYK et al (2014) Contemporary hearing rehabilitation options in patients with aural atresia. Biomed Res Int 2014:1–8. https://doi.org/10.1155/2014/761579
Yang J, Chen P, Zhao C et al (2020) Audiological and subjective outcomes of 100 implanted transcutaneous bone conduction devices and preoperative bone conduction hearing aids in patients with bilateral microtia-atresia. Acta Otolaryngol 140:667–673. https://doi.org/10.1080/00016489.2020.1762929
Irmer C, Volkenstein S, Dazert S, Neumann A (2022) The bone conduction implant BONEBRIDGE increases quality of life and social life satisfaction. Eur Arch Otorhinolaryngol 279:5555–5563. https://doi.org/10.1007/s00405-022-07384-w
Takano K, Takahashi N, Ogasawara N, Himi T (2016) The association of external and middle ear anomaly and mandibular morphology in congenital microtia. Otol Neurotol 37:889–894. https://doi.org/10.1097/MAO.0000000000001048
Plontke SK, Götze G, Wenzel C et al (2020) Implantation of a new active bone conduction hearing device with optimized geometry. HNO 68:106–115. https://doi.org/10.1007/s00106-020-00877-2
Yang J, Zhao C, Liu Y et al (2020) The effect of anatomical variables and use of the Lifts system on hearing outcomes after implantation of an active transcutaneous bone conduction device in bilateral congenital conductive hearing loss. J Otolaryngol Head Neck Surg 49:57. https://doi.org/10.1186/s40463-020-00452-3
Sprinzl GM, Schoerg P, Ploder M et al (2021) Surgical experience and early audiological outcomes with new active transcutaneous bone conduction implant. Otol Neurotol 42:1208–1215. https://doi.org/10.1097/MAO.0000000000003230
Kim H, Park MK, Park SN et al (2023) Efficacy of the Bonebridge BCI602 for adult patients with single-sided deafness: a prospective multicenter study. Otolaryngol Head Neck Surg. https://doi.org/10.1002/ohn.520
Zhao C, Yang J, Liu Y et al (2020) Horizontal sound localisation and speech perception in Bonebridge-implanted single-sided deafness patients. J Laryngol Otol 134:814–821. https://doi.org/10.1017/S0022215120001899
Rahne T, Schilde S, Seiwerth I et al (2016) Mastoid dimensions in children and young adults: consequences for the geometry of transcutaneous bone-conduction implants. Otol Neurotol 37:57–61. https://doi.org/10.1097/MAO.0000000000000881
Zernotti ME, Di Gregorio MF, Zernotti M (2021) Alternative inverted middle fossa approach in Bonebridge surgery. Technique results and complications. Int Arch Otorhinolaryngol 25:e374–e378. https://doi.org/10.1055/s-0040-1715152
Kong TH, Park YA, Seo YJ (2017) Image-guided implantation of the Bonebridge™ with a surgical navigation: a feasibility study. Int J Surg Case Rep 30:112–117. https://doi.org/10.1016/j.ijscr.2016.11.057
Law EKC, Bhatia KSS, Tsang WSS et al (2016) CT pre-operative planning of a new semi-implantable bone conduction hearing device. Eur Radiol 26:1686–1695. https://doi.org/10.1007/s00330-015-3983-x
Lassaletta L, Sanchez-Cuadrado I, Muñoz E, Gavilan J (2014) Retrosigmoid implantation of an active bone conduction stimulator in a patient with chronic otitis media. Auris Nasus Larynx 41:84–87. https://doi.org/10.1016/j.anl.2013.04.004
Šikolová S, Urík M, Hošnová D et al (2022) Two Bonebridge bone conduction hearing implant generations: audiological benefit and quality of hearing in children. Eur Arch Otorhinolaryngol 279:3387–3398. https://doi.org/10.1007/s00405-021-07068-x
Huber AM, Sim JH, Xie YZ et al (2013) The Bonebridge: preclinical evaluation of a new transcutaneously-activated bone anchored hearing device. Hear Res 301:93–99. https://doi.org/10.1016/j.heares.2013.02.003
Sprinzl G, Toner J, Koitschev A et al (2023) Multicentric study on surgical information and early safety and performance results with the Bonebridge BCI 602: an active transcutaneous bone conduction hearing implant. Eur Arch Otorhinolaryngol 280:1565–1579. https://doi.org/10.1007/s00405-022-07792-y
Yost WA (2016) Sound source localization identification accuracy: level and duration dependencies. J Acoust Soc Am 140:EL4–EL9. https://doi.org/10.1121/1.4954870
Acknowledgements
This work was supported by a Grant from the National Natural Science Foundation of China (no. 81770989) and the Capital Health Research and Development of Special (no. 2020-2-2057).
Author information
Authors and Affiliations
Contributions
Conceptualization: PC, SZ, DW, XF, RD; methodology: PC, LY, YL, JY; formal analysis and investigation: DW, RR, YL; writing—original draft preparation: PC; writing—review and editing: SZ; funding acquisition: SZ; resources: SZ, DW, XF, RD; supervision: DW.
Corresponding author
Ethics declarations
Conflict of interest
We have no financial disclosures that may pose or create a conflict of interest regarding the information presented in this article.
Ethical approval
Ethical approval for the execution of the present study was obtained from the Institutional Ethics Committee of Beijing Tongren Hospital, Capital Medical University (no. Z171100001017079).
Informed consent
All the participants signed an informed consent form before surgery.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, P., Liu, Y., Yang, J. et al. A new active bone-conduction implant: surgical experiences and audiological outcomes in patients with bilateral congenital microtia. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08523-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08523-1