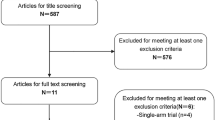
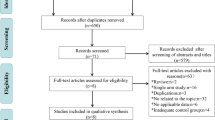
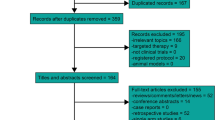
Abstract
Objective
To assess the safety and efficacy of gemcitabine and cisplatin (GP)‐based induction chemotherapy (IC) plus concurrent chemoradiotherapy (CCRT) for patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Methods
We systematically searched the PubMed, Web of Science, Embase, and Cochrane Library databases. The endpoints included overall survival (OS), progression-free survival (PFS), distant failure-free survival (DMFS), locoregional failure-free survival (LRFFS) and treatment-related adverse events (AEs).
Results
A total of seven studies were included in this meta-analysis. When GP-based IC was compared with double-drug-based or triple-drug-based IC, there were no significant differences in OS (HR 0.64, P = 0.08), PFS (HR 0.71, P = 0.09), DMFS (HR 0.87, P = 0.49) or LRFFS (HR 0.88, P = 0.66). Furthermore, subgroup analysis revealed that GP IC led to an improvement in OS compared with triple-drug-based IC (P < 0.0001). Regarding safety, compared to triple-drug-based IC, GP-based IC was related to a decreased risk of leucopenia (P = 0.007) and neutropenia (P = 0.02) but was associated with an increased risk of thrombocytopenia (P = 0.01). Compared to double-drug-based IC, the prevalence of grade 3 or above thrombocytopenia was higher in the GP group (P = 0.007). No significant difference in the incidence of other AEs was observed.
Conclusion
Based on efficacy and safety, our meta-analysis demonstrated that, compared to double-drug-based or triple-drug-based IC, IC with a GP regimen does not appear to improve OS, PFS, DMFS or LRFFS and mainly led to an increased risk of grade3/4 thrombocytopenia. Notably, our subgroup analysis data show that GP-based IC may bring improved trends in OS as compared to triple-drug-based IC. For the optimal IC regimen has not been established, which IC regimen will benefit most LA-NPC patients should be further explored.


Similar content being viewed by others
References
Chen YP, Chan ATC, Le QT et al (2019) Nasopharyngeal carcinoma. Lancet (London, England) 394(10192):64–80
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Ferlay J, Colombet M, Soerjomataram I et al (2018) Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 103:356–387
Chan AT, Teo PM, Johnson PJ (2002) Nasopharyngeal carcinoma. Ann Oncol 13(7):1007–1015
Guan H, He Y, Wei Z et al (2020) Assessment of induction chemotherapy regimen TPF vs GP followed by concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: A retrospective cohort study of 160 patients. Clin Otolaryngol 45:274–279
Liu M, You W, Song YB et al (2018) The changing role of chemotherapy in locoregionally advanced nasopharyngeal carcinoma: a up-dated systemic review and network meta-analysis. Front Oncol 8:597
Colevas AD, Yom SS, Pfister DG et al (2018) NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw 16:479–490
Liu T, Sun Q, Chen J et al (2018) A comparison of neoadjuvant chemotherapy with gemcitabine versus docetaxel plus cisplatin in locoregionally advanced nasopharyngeal carcinoma: a propensity score matching analysis. Cancer Manag Res 10:6237–6245
Zhang Y, Chen L, Hu GQ et al (2019) Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N Engl J Med 381(12):1124–1135
Frikha M, Auperin A, Tao Y et al (2018) A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006–02). Ann oncol 29(3):731–736
Hong RL, Hsiao CF, Ting LL et al (2018) Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann oncol 29(9):1972–1979
Li WF, Chen NY, Zhang N et al (2019) Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: long-term results of phase 3 randomized controlled trial. Int J cancer 145(1):295–305
Peng H, Chen L, Zhang J et al (2017) Induction chemotherapy improved long-term outcomes of patients with locoregionally advanced nasopharyngeal carcinoma: a propensity matched analysis of 5-year survival outcomes in the era of intensity-modulated radiotherapy. J Cancer 8:371–377
Ribassin-Majed L, Marguet S, Lee AWM et al (2017) What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. J Clin Oncol 35:498–505
Wang P, Zhang M, Ke C et al (2020) The efficacy and toxicity of induction chemotherapy plus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Medicine 99(10):e19360
Tan TH, Soon YY, Cheo T et al (2018) Induction chemotherapy for locally advanced nasopharyngeal carcinoma treated with concurrent chemoradiation: a systematic review and meta-analysis. Radiother Oncol 129(1):10–17
Wang M, Tian H, Li G et al (2016) Significant benefits of adding neoadjuvant chemotherapy before concurrent chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a meta-analysis of randomized controlled trials. Oncotarget 7:48375–48390
Ngan RK, Yiu HH, Lau WH et al (2002) Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol 13(8):1252–1258
Ma BB, Tannock IF, Pond GR et al (2002) Chemotherapy with gemcitabine- containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer 95(12):2516–2523
Wang J, Li J, Hong X et al (2008) Retrospective case series of gemcitabine plus cisplatin in the treatment of recurrent and metastatic nasopharyngeal carcinoma. Oral Oncol 44(5):464–470
Zhang L, Huang Y, Hong S et al (2016) Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 388:1883–1892
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (Prisma-P) 2015 statement. Syst Rev 4:1
Higgins JPT, López-López JA, Becker BJ et al (2019) Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health 4:e000858
Wells G, Shea B, O'Connell D et al (1999) The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Ottawa: Ottawa Health Research Institute 1999. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Wells GA, O’Connell D, Peterson J et al (2020) Newcastle-Ottawa quality assessment scale. http:// www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 5 May 2020
Higgins JPT et al (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://www.cochrane-handbook.org
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Smith GD, Schneider M et al (1997) Egger-Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Zhao L, Xu M, Jiang W et al (2017) Induction chemotherapy for the treatment of non-endemic locally advanced nasopharyngeal carcinoma. Oncotarget 8:6763–7677
Zang J, Xu M, Li C et al (2020) Gemcitabine and cisplatin versus docetaxel and cisplatin as induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma from non-endemic area of China. J Cancer Res Clin Oncol 146:2369–2378
Zeng Z, Yan RN, Tu L et al (2018) Assessment of concurrent chemoradiotherapy plus induction chemotherapy in advanced nasopharyngeal carcinoma: cisplatin, fluorouracil, and docetaxel versus gemcitabine and cisplatin. Sci Rep 8:15581
Yau TK, Lee AWM, Wong DHM et al (2006) Treatment of Stage IV(A-B) nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy and accelerated fractionation: impact of chemotherapy schemes. Int J Radiat Oncol Biol Phys 66:1004–1010
Huang YM, Qiao SQ, Lu L et al (2020) Gemcitabine combined with cisplatin vs. taxane, cisplatin, and fluorouracil in the treatment of locally advanced nasopharyngeal carcinoma: a retrospective case-control study. Eur Rev Med Pharmacol Sci 24:7655–7663
Zheng W, Qiu S, Huang L et al (2015) Is gemcitabine and cisplatin induction chemotherapy superior in locoregionally advanced nasopharyngeal carcinoma? Pak J Med Sci 31:781–786
Fangzheng W, Quanquan S, Chuner J et al (2017) Gemcitabine/cisplatin induction chemotherapy before concurrent chemotherapy and intensity-modulated radiotherapy improves outcomes for locoregionally advanced nasopharyngeal carcinoma. Oncotarget 8:96798–96808
Sun Y, Li WF, Chen NY et al (2016) Induction chemotherapy plus concur-rent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17(11):1509–1520
Kong L, Zhang Y, Hu C et al (2017) Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of 2 parallel phase 2 clinical trials. Cancer 123(12):2258–2267
Mikoshiba T, Ozawa H, Saito S et al (2019) Usefulness of hematological inflammatory markers in predicting severe side-effects from induction chemotherapy in head and neck cancer patients. Anticancer Res 39:3059–3065
Kang RY, Yoo KS, Han HJ et al (2017) Evaluation of the effects and adverse drug reactions of low-dose dexamethasone premedication with weekly docetaxel. Support Care Cancer 25:429–437
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by, MT, ZJ and JZ. The first draft of the manuscript was written by MT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Ethics approval
Not applied.
Consent to participate
Not applied.
Consent for publication
Not applied.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhongxiong Jia is a co-first author.
Rights and permissions
About this article
Cite this article
Tang, M., Jia, Z. & Zhang, J. The safety and efficacy of gemcitabine and cisplatin (GP)‐based induction chemotherapy plus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a meta‐analysis. Eur Arch Otorhinolaryngol 279, 1561–1572 (2022). https://doi.org/10.1007/s00405-021-06940-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06940-0