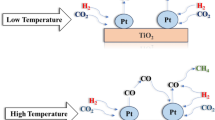
Abstract
The enormous rise in population, urbanization, industrialization, and improvement in people’s living conditions over the past few decades have led to an increase in the consumption of fossil fuels. While there are several strategies to overcome it, such as afforestation, increasing the number of natural sinks, trapping the produced carbon dioxide in exhausted oil wells, and sorption of CO2 on absorbers, all of these are only short-term remedies. However, a long-term solution would be conversion of CO2 into another type of substance. Thereby, reduction of CO2 to generate short chain hydrocarbons like CH3OH, CH4, C2H6, etc. can act as a renewable strategy to produce green energy sources. Reduction of CO2 can be achieved following several pathways which include pyrolysis, chemical-catalysis, electrocatalysis and/or photocatalysis, etc. Among these routes, photocatalytic pathway is more appealing as it is an inherently green approach where solar light can directly convert CO2 to value-added products. During molecular photocatalytic CO2 reduction, typically Ru- or Ir-based photosensitizers are commonly used in presence of a cocatalyst. In this context, metal chalcogenides photocatalysts including CdS, CuS, MoS2, ZnS, ZnTe, and their heterostructures like CdSe/CdS, CdS/CeO2, etc. are made of earth-abundant metals and have received a considerable attention towards CO2 reduction due to their notable photocatalytic efficiency. As it is well known, it is nearly impossible to concurrently satisfy the various performances in a one-step excitation system utilizing a single photocatalyst. As a result, the idea of composite photocatalysts which combine the advantages of many materials emerged, and this trend has become essential and foreseeable. This compact review highlights the application and the engagement of metal chalcogenides which due to their unique properties not only helps in the reduction of CO2 but also plays a vital role in reducing environmental pollution and hence seen as a potential remedy towards the concerned problem.
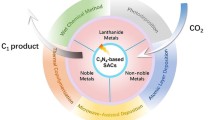
Graphical Abstract
This compact review highlights the application and engagement of metal chalcogenides which due to their unique properties not only helps in the reduction of CO2 but also plays a vital role in reducing environmental pollution. Various metal chalcogenides photocatalysts including CdS, CuS, MoS2, ZnS, ZnTe, and their heterostructures like CdSe/CdS, CdS/CeO2, etc. are made of earth-abundant metals and have received a considerable attention towards CO2 reduction due to their notable photocatalytic efficiency.



© 2021 Wiley–VCH GmbH)



Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Anpo M (2013) Photocatalytic reduction of CO2 with H2O on highly dispersed Ti-oxide catalysts as a model of artificial photosynthesis. J CO2 Util 1:8–17. https://doi.org/10.1016/j.jcou.2013.03.005
Kothandaraman J, Heldebrant DJ (2020) Towards environmentally benign capture and conversion: heterogeneous metal catalyzed CO2 hydrogenation in CO2 capture solvents. Green Chem 22(3):828–834. https://doi.org/10.1039/C9GC03449H
Khan AA, Tahir M (2019) Recent advancements in engineering approach towards design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J CO2 Util 29:205–239. https://doi.org/10.1016/j.jcou.2018.12.008
Ebrahimi A, Sarrafi A, Tahmooresi M (2017) Methane and hydrogen production from carbon dioxide by ZnS, CuS, and CuS doped ZnS nanophotocatalyst deposited on montmorillonite. Energy Sources, Part A: Recovery, Utilization and Environmental Effects 39(7):680–686. https://doi.org/10.1080/15567036.2016.1255678
Wu J, Huang Y, Ye W, Li Y, Wu J, Huang Y, Ye W, Li Y (2017) CO2 reduction: from the electrochemical to photochemical approach. Adv Sci 4(11):1700194. https://doi.org/10.1002/advs.201700194
Peter SC (2018) Reduction of CO2 to chemicals and fuels: a solution to global warming and energy crisis. ACS Energy Lett 3(7):1557–1561. https://doi.org/10.1021/acsenergylett.8b00878
Yang CC, Yu YH, Van Der Linden B, Wu JCS, Mul G (2010) Artificial photosynthesis over crystalline TiO2-based catalysts: fact or fiction? J Am Chem Soc 132(24):8398–8406. https://doi.org/10.1021/ja101318k
Hegazy IM, Geioushy RA, El-Sheikh SM, Shawky A, Kandil A-HT (2020) Influence of oxygen vacancies on the performance of ZnO nanoparticles towards CO2 photoreduction in different aqueous solutions. J Environ Chem Eng 8(4):103887. https://doi.org/10.1016/j.jece.2020.103887
Zeng S, Kar P, Thakur UK, Shankar K (2018) A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials. Nanotechnology 29(5):052001. https://doi.org/10.1088/1361-6528/aa9fb1
Yang K, Yang Z, Zhang C, Gu Y, Wei J, Li Z, Ma C, Yang X, Song K, Li Y, Fang Q, Zhou J (2021) Recent advances in CdS-based photocatalysts for CO2 photocatalytic conversion. J Chem Eng 418. https://doi.org/10.1016/j.crcon.2020.02.003
Lin J, Tian W, Zhang H, Duan X, Sun H, Wang S (2021) Graphitic carbon nitride-based Z-scheme structure for photocatalytic CO2 reduction. Energy Fuels 35(1):7–24. https://doi.org/10.1021/acs.energyfuels.0c03048
Gusain R, Kumar P, Sharma OP, Jain SL, Khatri OP (2016) Reduced graphene oxide-CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl Catal B: Environ 181:352–362. https://doi.org/10.1016/j.apcatb.2015.08.012
Yang ZZ, Wei JJ, Zeng GM, Zhang HQ, Tan XF, Ma C, Li XC, Li ZH, Zhang C (2019) A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord Chem Rev 386:154–182. https://doi.org/10.1016/j.ccr.2019.01.018
Otgonbayar Z, Oh WC (2023) Bandgap energy controlling of quaternary metal sulfide-graphene-ZnO ternary nanocomposite for photocatalytic reduction of CO2. Sep Purif Technol 324:124522. https://doi.org/10.1016/j.seppur.2023.124522
Pedersen PD, Vegge T, Bligaard T, Hansen HA (2023) Trends in CO2 reduction on transition metal dichalcogenide edges. ACS Catal 13(4):2341–2350. https://doi.org/10.1021/acscatal.2c04825
Geng X, Liu D, Hewa-Rahinduwage CC, Brock SL, Luo L (2023) Electrochemical gelation of metal chalcogenide quantum dots: applications in gas sensing and photocatalysis. Acc Chem Res 56(9):1087–1096. https://doi.org/10.1021/acs.accounts.3c00042
El-Gendy RA, El-Bery HM, Farrag M, Fouad DM (2023) Metal chalcogenides (CuS or MoS2)-modified TiO2 as highly efficient bifunctional photocatalyst nanocomposites for green H2 generation and dye degradation. Sci Rep 13(1):1–15. https://doi.org/10.1038/s41598-023-34743-2
Pramoda K, Rao CNR (2023) 2D transition metal-based phospho-chalcogenides and their applications in photocatalytic and electrocatalytic hydrogen evolution reactions. J Mater Chem A 11(32):16933–16962. https://doi.org/10.1039/D3TA01629C
Munonde TS, Nomngongo PN (2023) Review on metal chalcogenides and metal chalcogenide-based nanocomposites in photocatalytic applications. Chem Afr 6(3):1127–1143. https://doi.org/10.1007/s42250-022-00577-0
Domyati D (2023) Chemical and thermal study of metal chalcogenides (zinc sulfide), aluminum oxide, and graphene oxide based nanocomposites for wastewater treatment. Ceram Int 49(1):1464–1472. https://doi.org/10.1016/j.ceramint.2022.09.190
Chang X, Wang T, Gong J (2016) CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ Sci 9(7):2177–2196. https://doi.org/10.1039/C6EE00383D
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed 52(29):7372–7408. https://doi.org/10.1002/anie.201207199
Mao J, Li K, Peng T (2013) Recent advances in the photocatalytic CO2 reduction over semiconductors. Catal Sci Technol 3(10):2481–2498. https://doi.org/10.1039/C3CY00345K
Arora I, Chawla H, Chandra A, Sagadevan S, Garg S (2022) Advances in the strategies for enhancing the photocatalytic activity of TiO2: conversion from UV-light active to visible-light active photocatalyst. Inorg Chem Commun 143:109700. https://doi.org/10.1016/j.inoche.2022.109700
Raula M, Ganor G, Saganovich M, Zeiri O, Wang Y, Chierotti MR, Gobetto R, Weinstock IA (2015) Polyoxometalate complexes of anatase-titanium dioxide cores in water. Angew Chem Int Ed 54(42):12416–12421. https://doi.org/10.1002/anie.201501941
Chakraborty B, Gan-Or G, Duan Y, Raula M, Weinstock IA (2019) Visible-light-driven water oxidation with a polyoxometalate-complexed hematite core of 275 iron atoms. Angew Chem Int Ed 58(20):6584–6589. https://doi.org/10.1002/anie.201900492
Saganovich M, Gadot E, Raula M, Weinstock IA (2018) Proton-coupled electron transfer from photo-excited CdS nanoparticles. J Coord Chem 71(11–13):2012–2014. https://doi.org/10.1080/00958972.2018.1487556
Chakraborty B, Gan-Or G, Raula M, Gadot E, Weinstock IA (2018) Design of an inherently-stable water oxidation catalyst. Nat Commun 9(1):4896. https://doi.org/10.1038/s41467-018-07281-z
Gupta TK, Sengupta S, Raula M (2022) Optimization of process, mechanism and kinetics study for photocatalytic oxidation. Green Photocatalytic Semicon 33–48. https://doi.org/10.1007/978-3-030-77371-7_20
Shi J, Wang X, Zhang S, Xiao L, Huan Y, Gong Y, Zhang Z, Li Y, Zhou X, Hong M, Fang Q, Zhang Q, Liu X, Gu L, Liu Z, Zhang Y (2017) Two-dimensional metallic tantalum disulfide as a hydrogen evolution catalyst. Nat Commun 8:958. https://doi.org/10.1038/s41467-017-01089-z
He LP, Hong XC, Dong JK, Pan J, Zhang Z, Zhang J, Li SY (2014) Quantum transport evidence for the three-dimensional dirac semimetal phase in Cd3As2. Phys Rev Lett 113(24):246402. https://doi.org/10.1103/PhysRevLett.113.246402
Britnell L, Ribeiro RM, Eckmann A, Jalil R, Belle BD, Mishchenko A, Kim YJ, Gorbachev RV, Georgiou T, Morozov SV, Grigorenko AN, Geim AK, Casiraghi C, Castro Neto AH, Novoselov KS (2013) Strong light-matter interactions in heterostructures of atomically thin films. Sci 340:1311–1314. https://doi.org/10.1126/science.1235547
Balendhran S, Walia S, Nili H, Ou JZ, Zhuiykov S, Kaner RB, Sriram S, Bhaskaran M, Kalantar-Zadeh K (2013) Two-dimensional molybdenum trioxide and dichalcogenides. Adv Funct Mater 23:3952–3970. https://doi.org/10.1002/adfm.201300125
Zeng H, Liu GB, Dai J, Yan Y, Zhu B, He R, Xie L, Xu S, Chen X, Yao W, Cui X (2013) Optical signature of symmetry variations and spin-valley coupling in atomically thin tungsten dichalcogenides. Sci Rep 3:1608. https://doi.org/10.1038/srep01608
Ogawa A (2000) Activation and reactivity of group 16 inter-element linkage - transition-metal-catalyzed reactions of thiols and selenols. J Organomet Chem 611:463–474. https://doi.org/10.1016/S0022-328X(00)00469-1
Khalafallah D, Qiao F, Liu C, Wang J, Zhang Y, Wang J, Zhang Q, Notten PHL (2023) Heterostructured transition metal chalcogenides with strategic heterointerfaces for electrochemical energy conversion/storage. Coord Chem Rev 496:215405. https://doi.org/10.1016/j.ccr.2023.215405
Zhao B, Shen D, Zhang Z, Lu P, Hossain M, Li J, Li B, Duan X (2021) 2D metallic transition-metal dichalcogenides: structures, synthesis, properties, and applications. Adv Funct Mater 31(48):2105132. https://doi.org/10.1002/adfm.202105132
Siddiqui MS, Aslam M (2021) Chalcogenides as well as chalcogenides-based nanomaterials and its importance in photocatalysis. Micro Nano Technol Elsevier. https://doi.org/10.1016/B978-0-12-820498-6.00003-2
Muslih EY, Munir B, Khan MM (2021) Advances in chalcogenides and chalcogenides-based nanomaterials such as sulfides, selenides, and tellurides. Micro Nano Technol. https://doi.org/10.1016/B978-0-12-820498-6.00002-0
Xia D, Chen Q, Li Z, Luo M, Wong PK (2021) Band gap engineered chalcogenide nanomaterials for visible light-induced photocatalysis. Micro Nano Technol. https://doi.org/10.1016/B978-0-12-820498-6.00006-8
Haque F, Daeneke T, Kalantar-zadeh K, Ou JZ (2017) Two-dimensional transition metal oxide and chalcogenide-based photocatalysts. Nano-Micro Letters 10(2):1–27. https://doi.org/10.1007/s40820-017-0176-y
Shao X, Zhang X, Liu Y, Qiao J, Zhou XD, Xu N, Malcombe JL, Yi J, Zhang J (2021) Metal chalcogenide-associated catalysts enabling CO2 electroreduction to produce low-carbon fuels for energy storage and emission reduction: catalyst structure, morphology, performance, and mechanism. J Mater Chem A 9(5):2526–2559. https://doi.org/10.1039/D0TA09232K
Ali SA, Sadiq I, Ahmad T (2023) Oxide based heterostructured photocatalysts for CO2 reduction and hydrogen generation. ChemistrySelect 8(8):e202203176. https://doi.org/10.1002/slct.202203176
Li K, Peng B, Peng T (2016) Recent advances in heterogeneous photocatalytic CO2 conversion to solar fuels. ACS Catal 6(11):7485–7527. https://doi.org/10.1021/acscatal.6b02089
Hiragond CB, Powar NS, In SI (2020) Recent developments in lead and lead-free halide perovskite nanostructures towards photocatalytic CO2 reduction. Nanomaterials 10(12):2569. https://doi.org/10.3390/nano10122569
Schneider J, Jia H, Muckerman JT, Fujita E (2012) Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem Soc Rev 41(6):2036–2051. https://doi.org/10.1039/C1CS15278E
Gattrell M, Gupta N, Co A (2006) A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J Electroanal Chem 594(1):1–19. https://doi.org/10.1016/j.jelechem.2006.05.013
He H, Zapol P, Curtiss LA (2012) Computational screening of dopants for photocatalytic two-electron reduction of CO2 on anatase (101) surfaces. Energy Environ Sci 5(3):6196–6205. https://doi.org/10.1039/C2EE02665A
Subrahmanyam M, Kaneco S, Alonso-Vante N (1999) A screening for the photo reduction of carbon dioxide supported on metal oxide catalysts for C1–C3 selectivity. Appl Catal B 23(2–3):169–174. https://doi.org/10.1016/S0926-3373(99)00079-X
Sasirekha N, Basha SJS, Shanthi K (2006) Photocatalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide. Appl Catal B 62(1–2):169–180. https://doi.org/10.1016/j.apcatb.2005.07.009
Anpo M, Yamashita H, Ichihashi Y, Ehara S (1995) Photocatalytic reduction of CO2 with H2O on various titanium oxide catalysts. J Electroanal Chem 396(1–2):21–26. https://doi.org/10.1016/0022-0728(95)04141-A
Tan SS, Zou L, Hu E (2008) Kinetic modelling for photosynthesis of hydrogen and methane through catalytic reduction of carbon dioxide with water vapour. Catal Today 131(1–4):125–129. https://doi.org/10.1016/j.cattod.2007.10.011
Dey GR, Belapurkar AD, Kishore K (2004) Photo-catalytic reduction of carbon dioxide to methane using TiO2 as suspension in water. J Photochem Photobiol A Chem 163(3):503–508. https://doi.org/10.1016/j.jphotochem.2004.01.022
Dey GR, Pushpa KK (2007) Formation of different products during photo-catalytic reaction on TiO2 suspension in water with and without 2-propanol under diverse ambient conditions. Res Chem Intermed 33(7):631–644. https://doi.org/10.1163/156856707781749883/METRICS
Sato S (1983) Photo-Kolbe reaction at gas-solid interfaces. J Phys Chem 87(18):3531–3537. https://doi.org/10.1021/J100241A034
Ward MD, White JR, Bard AJ (1983) Electrochemical investigation of the energetics of particulate titanium dioxide photocatalysts. The Methyl Viologen-Acetate System. J Am Chem Soc 105(1):27–31. https://doi.org/10.1021/JA00339A007/ASSET/JA00339A007.FP.PNG_V03
Kaise M, Kondoh H, Nishihara C, Nozoye H, Shindo H, Nimura S, Kikuchi O (1993) Photocatalytic reactions of acetic acid on platinum-loaded TiO2: ESR evidence of radical intermediates in the photo-Kolbe reaction. J Chem Soc Chem Commun 4:395–396. https://doi.org/10.1039/C39930000395
Mota FM, Kim DH (2019) From CO2 methanation to ambitious long-chain hydrocarbons: alternative fuels paving the path to sustainability. Chem Soc Rev 48(1):205–259. https://doi.org/10.1039/C8CS00527C
Yanagida S, Kanemoto M, Ishihara KI, Wada Y, Sakata T, Mori H (2006) Visible-light induced photoreduction of CO2 with CdS nanocrystallites — importance of the morphology and surface structures controlled through solvation by N, N-dimethylformamide. Bull Chem Soc Jpn 70(9):2063–2070. https://doi.org/10.1039/c3dt53601g
Henglein A (1982) Photo-degradation and fluorescence of colloidal-cadmium sulfide in aqueous solution. Ber Bunsen-Ges-Phys Chem Chem Phys 86(4):301–305. https://doi.org/10.1002/bbpc.19820860409
Pelouch WS, Ellingson RJ, Powers PE, Tang CL, Szmyd DM, Nozik AJ (1992) Comparison of hot-carrier relaxation in quantum wells and bulk GaAs at high carrier densities. Phys Rev B 45(3):1450. https://doi.org/10.1103/PhysRevB.45.1450
Wu J, Liu J, Xia W, Ren YY, Wang F (2021) Advances on photocatalytic CO2 reduction based on CdS and CdSe nano-semiconductors. J Chin Chem Soc. https://doi.org/10.3866/PKU.WHXB202008043
Wu K, Lian T (2016) Quantum confined colloidal nanorod heterostructures for solar-to-fuel conversion. Chem Soc Rev 45(14):3781–3810. https://doi.org/10.1039/C5CS00472A
Panfil YE, Oded M, Banin U (2018) Colloidal quantum nanostructures: emerging materials for display applications. Angew Chem Int Ed 57(16):4274–4295. https://doi.org/10.1002/anie.201708510
Wang HY, Hu R, Lei YJ, Jia ZY, Hu GL, Li CB, Gu Q (2020) Highly efficient and selective photocatalytic CO2 reduction based on water-soluble CdS QDs modified by the mixed ligands in one pot. Catal Sci Technol 10(9):2821–2829. https://doi.org/10.1039/D0CY00308E
Xiang X, Wang L, Zhang J, Cheng B, Yu J, Macyk W (2022) Cadmium chalcogenide (CdS, CdSe, CdTe) quantum dots for solar-to-fuel conversion. Adv photonics 3(11):2200065. https://doi.org/10.1002/adpr.202200065
Wang F, Hou T, Zhao X, Yao W, Fang R, Shen K, Li Y (2021) Ordered macroporous carbonous frameworks implanted with CdS quantum dots for efficient photocatalytic CO2 reduction. Adv Mater 33(35):2102690. https://doi.org/10.1002/adma.202102690
Yu J, Jin J, Cheng B, Jaroniec M (2014) A noble metal-free reduced graphene oxide–CdS nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J Mater Chem A 2(10):3407–3416. https://doi.org/10.1039/C3TA14493C
Feng Y, Yan X, Liu C, Hong Y, Zhu L, Zhou M, Shi W (2015) Hydrothermal synthesis of CdS/Bi2MoO6 heterojunction photocatalysts with excellent visible-light-driven photocatalytic performance. Appl Surf Sci 353:87–94. https://doi.org/10.1016/j.apsusc.2015.06.061
Ijaz S, Ehsan MF, Ashiq MN, Karamat N, He T (2016) Preparation of CdS@CeO2 core/shell composite for photocatalytic reduction of CO2 under visible-light irradiation. Appl Surf Sci 390:550–559. https://doi.org/10.1016/j.apsusc.2016.08.098
Wang J, Lin S, Tian N, Ma T, Zhang Y, Huang H (2020) Nanostructured metal sulfides: classification, modification strategy, and solar-driven CO2 reduction application. Adv Funct Mater 31(9):2008008. https://doi.org/10.1002/adfm.202008008
Adachi K, Ohta K, Mizuno T (1994) Photocatalytic reduction of carbon dioxide to hydrocarbon using copper-loaded titanium dioxide. Sol Energy 53(2):187–190. https://doi.org/10.1016/0038-092X(94)90480-4
Kočí K, Matějová L, Kozák O, Čapek L, Valeš V, Reli M, Praus P, Šafářová K, Kotarba A, Obalová L (2014) ZnS/MMT nanocomposites: the effect of ZnS loading in MMT on the photocatalytic reduction of carbon dioxide. Appl Catal B: Environ 158–159:410–417. https://doi.org/10.1016/j.apcatb.2014.04.048
Xu F, Zhu B, Cheng B, Yu J, Xu J (2018) 1D/2D TiO2/MoS2 hybrid nanostructures for enhanced photocatalytic CO2 reduction. Adv Opt Mater 6(23):1800911. https://doi.org/10.1002/adom.201800911
Tu W, Li Y, Kuai L, Zhou Y, Xu Q, Li H, Wang X, Xiao M, Zou Z (2017) Construction of unique two-dimensional MoS2–TiO2 hybrid nanojunctions: MoS2 as a promising cost-effective cocatalyst toward improved photocatalytic reduction of CO2 to methanol. Nanoscale 9(26):9065–9070. https://doi.org/10.1039/C7NR03238B
Tian N, Huang H, He Y, Guo Y, Zhang T, Zhang Y (2015) Mediator-free direct Z-scheme photocatalytic system: BiVO4/g-C3N4 organic–inorganic hybrid photocatalyst with highly efficient visible-light-induced photocatalytic activity. Dalton Trans 44(9):4297–4307. https://doi.org/10.1039/C4DT03905J
Low J, Cheng B, Yu J, Jaroniec M (2016) Carbon-based two-dimensional layered materials for photocatalytic CO2 reduction to solar fuels. Energy Stor Mater 3:24–35. https://doi.org/10.1016/j.ensm.2015.12.003
Zhu B, Xia P, Ho W, Yu J (2015) Isoelectric point and adsorption activity of porous G-C3N4. Appl Surf Sci 344:188–195. https://doi.org/10.1016/j.apsusc.2015.03.086
Zhu M, Zhai C, Sun M, Hu Y, Yan B, Du Y (2017) Ultrathin graphitic C3N4 nanosheet as a promising visible-light-activated support for boosting photoelectrocatalytic methanol oxidation. Appl Catal B: Environ 203:108–115. https://doi.org/10.1016/j.apcatb.2016.10.012
Zhang YC, Du ZN, Li KW, Zhang M, Dionysiou DD (2011) High-performance visible-light-driven SnS2/SnO2 nanocomposite photocatalyst prepared via in situ hydrothermal oxidation of SnS2 nanoparticles. ACS Appl Mater Interfaces 3(5):1528–1537. https://doi.org/10.1021/am200102y
Yu J, Xu CY, Ma FX, Hu SP, Zhang YW, Zhen L (2014) Monodisperse SnS2 nanosheets for high-performance photocatalytic hydrogen generation. ACS Appl Mater Interfaces 6(24):22370–22377. https://doi.org/10.1021/am506396z
An X, Yu JC, Tang J (2013) Biomolecule-assisted fabrication of copper doped SnS2 nanosheet–reduced graphene oxide junctions with enhanced visible-light photocatalytic activity. J Mater Chem A 2(4):1000–1005. https://doi.org/10.1039/C3TA13846A
Di T, Zhu B, Cheng B, Yu J, Xu J (2017) A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J Catal 352:532–541. https://doi.org/10.1016/j.jcat.2017.06.006
Zhang YC, Li J, Xu HY (2012) One-step in situ solvothermal synthesis of SnS2/TiO2 nanocomposites with high performance in visible light-driven photocatalytic reduction of aqueous Cr(VI). Appl Catal B: Environ 123–124:18–26. https://doi.org/10.1016/j.apcatb.2012.04.018
She H, Zhou H, Li L, Zhao Z, Jiang M, Huang J, Wang L, Wang Q (2019) Construction of a two-dimensional composite derived from TiO2 and SnS2 for enhanced photocatalytic reduction of CO2 into CH4. ACS Sustain Chem Eng 7(1):650–659. https://doi.org/10.1021/acssuschemeng.8b04250
Han A, Li M, Zhang S, Zhu X, Han J, Ge Q, Wang H (2019) Ti3+ defective SnS2/TiO2 heterojunction photocatalyst for visible-light driven reduction of CO2 to CO with high selectivity. Catalysts 9(11):927. https://doi.org/10.3390/catal9110927
Mumin MA, Moula G, Charpentier PA (2015) Supercritical CO2 synthesized TiO2 nanowires covalently linked with core–shell CdS–ZnS quantum dots: enhanced photocatalysis and stability. RSC Adv 5(83):67767–67779. https://doi.org/10.1039/C5RA08914J
Li YS, Jiang FL, Xiao Q, Li R, Li K, Zhang MF, Zhang AQ, Sun SF, Liu Y (2010) Enhanced photocatalytic activities of TiO2 nanocomposites doped with water-soluble mercapto-capped CdTe quantum dots. Appl Catal B: Environ 101(1–2):118–129. https://doi.org/10.1016/j.apcatb.2010.09.016
Yu J, Zhang J, Jaroniec M (2010) Preparation and enhanced visible-light photocatalytic H2-production activity of CdS quantum dots-sensitized Zn1−xCdxS solid solution. Green Chem 12(9):1611–1614. https://doi.org/10.1039/C0GC00236D
Chen J, Xin F, Niu H, Mao CJ, Song JM (2017) Photocatalytic reduction of CO2 with methanol over Bi2S3-ZnIn2S4 nanocomposites. Mater Lett 198:1–3. https://doi.org/10.1016/j.matlet.2017.03.164
Guo RT, Liu XY, Qin H, Wang ZY, Shi X, Pan WG, Fu ZG, Tang JY, Jia PY, Miao YF, Gu JW (2020) Photocatalytic reduction of CO2 into CO over nanostructure Bi2S3 quantum dots/g-C3N4 composites with Z-scheme mechanism. Appl Surf Sci 500
Ijaz S, Ehsan MF, Ashiq MN, He T (2015) Synthesis of a Bi2S3/CeO2 nanocatalyst and its visible light-driven conversion of CO2 into CH3OH and CH4. Catal Sci Technol 5(12):5208–5215. https://doi.org/10.1039/C5CY00955C
Li X, Liu H, Luo D, Li J, Huang Y, Li H, Fang Y, Xu Y, Zhu L (2012) Adsorption of CO2 on heterostructure CdS(Bi2S3)/TiO2 nanotube photocatalysts and their photocatalytic activities in the reduction of CO2 to methanol under visible light irradiation. J Chem Eng 180:151–158. https://doi.org/10.1016/j.cej.2011.11.029
Aliwi SM, Al-Jubori KF (1989) Photoreduction of CO2 by metal sulphide semiconductors in presence of H2S. Sol Energy Mater Sol 18(3–4):223–229. https://doi.org/10.1016/0165-1633(89)90056-7
Li X, Chen J, Li H, Li J, Xu Y, Liu Y, Zhou J (2011) Photoreduction of CO2 to methanol over Bi2S3/CdS photocatalyst under visible light irradiation. J Nat Gas Chem 20(4):413–417. https://doi.org/10.1016/S1003-9953(10)60212-5
Soci C, Zhang A, Xiang B, Dayeh SA, Aplin DPR, Park J, Bao XY, Lo YH, Wang D (2007) ZnO nanowire UV photodetectors with high internal gain. Nano Lett 7(4):1003–1009. https://doi.org/10.1021/nl070111x
Soci C, Hwang IW, Moses D, Zhu Z, Waller D, Gaudiana R, Brabec CJ, Heeger AJ (2007) Photoconductivity of a low-bandgap conjugated polymer. Adv Funct Mater 17(4):632–636. https://doi.org/10.1002/adfm.200600199
Wang P, Huang B, Zhang X, Qin X, Dai Y, Jin H, Wei J, Whangbo MH (2008) Composite semiconductor H2WO4⋅H2O/AgCl as an efficient and stable photocatalyst under visible light. Chem Eur J 14(34):10543–10546
Vattikuti SVP, Shim J, Byon C (2018) 1D Bi2S3 nanorod/2D e-WS2 nanosheet heterojunction photocatalyst for enhanced photocatalytic activity. J Solid State Chem 258:526–535. https://doi.org/10.1016/j.jssc.2017.11.017
Xiao P, Lou J, Zhang H, Song W, Wu XL, Lin H, Chen J, Liu S, Wang X (2018) Enhanced visible-light-driven photocatalysis from WS2 quantum dots coupled to BiOCl nanosheets: synergistic effect and mechanism insight. Catal Sci Technol 8(1):201–209. https://doi.org/10.1039/C7CY01784G
Qi J, Xiong H, Wang G, Xie H, Jia W, Zhang Q, Li Y, Wang H (2018) High-performance solar cells with induced crystallization of perovskite by an evenly distributed CdSe quantum dots seed-mediated underlayer. J Power Sources 376:46–54. https://doi.org/10.1016/j.jpowsour.2017.11.062
Ren N, Yang ZJ, Lv XC, Shi J, Zhang YH, Tang Y (2010) A seed surface crystallization approach for rapid synthesis of submicron ZSM-5 zeolite with controllable crystal size and morphology. Microporous Mesoporous Mater 131(1–3):103–114. https://doi.org/10.1016/j.micromeso.2009.12.009
Ge ZH, Zhang BP, Shang PP, Li JF (2011) Control of anisotropic electrical transport property of Bi2S3 thermoelectric polycrystals. J Mater Chem 21(25):9194–9200. https://doi.org/10.1039/C1JM11069A
Dai W, Yu J, Luo S, Hu X, Yang L, Zhang S, Li B, Luo X, Zou J (2020) WS2 quantum dots seeding in Bi2S3 nanotubes: a novel vis-NIR light sensitive photocatalyst with low-resistance junction interface for CO2 reduction. Int J Hydrog Energy 389:123430. https://doi.org/10.1016/j.cej.2019.123430
Wang Y, Zhang Z, Zhang L, Luo Z, Shen J, Lin H, Long J, Wu JCS, Fu X, Wang X, Li C (2018) Visible-light driven overall conversion of CO2 and H2O to CH4 and O2 on 3D-SiC@2D-MoS2 heterostructure. J Am Chem Soc 140(44):14595–14598. https://doi.org/10.1021/jacs.8b09344
Balan B, Xavier MM, Mathew S (2023) MoS2-based nanocomposites for photocatalytic hydrogen evolution and carbon dioxide reduction. ACS Omega 8(29):25649–25673. https://doi.org/10.1021/acsomega.3c02084
Li A, Wang T, Li C, Huang Z, Luo Z, Gong J (2019) Adjusting the reduction potential of electrons by quantum confinement for selective photoreduction of CO2 to methanol. Angew Chem Int Ed 58(12):3804–3808. https://doi.org/10.1002/anie.201812773
Fujiwara H, Hosokawa H, Murakoshi K, Wada Y, Yanagida S, Okada T, Kobayashi H (1997) Effect of surface structures on photocatalytic CO2 reduction using quantized CdS nanocrystallites1. J Phys Chem B 101(41):8270–8278. https://doi.org/10.1021/jp971621q
Guo Q, Liang F, Li XB, Gao YJ, Huang MY, Wang Y, Xia SG, Gao XY, Gan QC, Lin ZS, Tung CH, Wu LZ (2019) Efficient and selective CO2 reduction integrated with organic synthesis by solar energy. Chem 5(10):2605–2616. https://doi.org/10.1016/j.chempr.2019.06.019
Wang C, Kwon KW, Odlyzko ML, Lee BH, Shim M (2007) PbSe nanocrystal/TiOx heterostructured films: a simple route to nanoscale heterointerfaces and photocatalysis. Phys Chem C 111(31):11734–11741. https://doi.org/10.1021/jp073022h
Wang C, Thompson RL, Baltrus J, Matranga C (2010) Visible light photoreduction of CO2 using CdSe/Pt/TiO2 heterostructured catalysts. J Phys Chem Lett 1(1):48–53. https://doi.org/10.1021/jz9000032
Xia W, Wu J, Hu JC, Sun S, Li MD, Liu H, Lan M, Wang F (2019) Highly efficient photocatalytic conversion of CO2 to CO catalyzed by surface-ligand-removed and Cd-rich CdSe quantum dots. ChemSusChem 12(20):4617–4622. https://doi.org/10.1002/cssc.201901633
Sheng H, Oh MH, Osowiecki WT, Kim W, Alivisatos AP, Frei H (2018) Carbon dioxide dimer radical anion as surface intermediate of photoinduced CO2 reduction at aqueous Cu and CdSe nanoparticle catalysts by rapid-scan FT-IR spectroscopy. J Am Chem Soc 140(12):4363–4371. https://doi.org/10.1021/jacs.8b00271
Kaniyankandy S, Rawalekar S, Verma S, Ghosh HN (2011) Ultrafast hole transfer in CdSe/ZnTe type II core-shell nanostructure. J Phys Chem 115(5):1428–1435. https://doi.org/10.1021/jp107531h
Wang Y, He T (2019) ZnTe-based nanocatalysts for CO2 reduction. Curr Opin Green Sustain Chem 16:7–12. https://doi.org/10.1016/j.cogsc.2018.11.005
Ehsan MF, Ashiq MN, Bi F, Bi Y, Palanisamy S, He T (2014) Preparation and characterization of SrTiO3–ZnTe nanocomposites for the visible-light photoconversion of carbon dioxide to methane. RSC Adv 4(89):48411–48418. https://doi.org/10.1039/C4RA06828A
Li P, Luo G, Zhu S, Guo L, Qu P, He T (2020) Unraveling the selectivity puzzle of H2 evolution over CO2 photoreduction using ZnS nanocatalysts with phase junction. Appl Catal B: Environ 274:119115. https://doi.org/10.1016/j.apcatb.2020.119115
Zhang Y, Wu Y, Wan L, Ding H, Li H, Wang X, Zhang W (2022) Hollow core–shell Co9S8@ZnIn2S4/CdS nanoreactor for efficient photothermal effect and CO2 photoreduction. Appl Catal B: Environ 311:121255. https://doi.org/10.1016/j.apcatb.2022.121255
Wang HY, Xie WH, Wei DD, Hu R, Wang N, Chang K, Lei SL, Wang B, Cao R (2022) A hybrid assembly with nickel poly-pyridine polymer on CdS quantum dots for photo-reducing CO2 into syngas with controlled H2/CO ratios. Chemsuschem 15(8):e202200200. https://doi.org/10.1002/cssc.202200200
Fu J, Jiang K, Qiu X, Yu J, Liu M (2020) Product selectivity of photocatalytic CO2 reduction reactions. Mater Today 32:222–243. https://doi.org/10.1016/j.mattod.2019.06.009
Dilla M, Mateblowski A, Ristig S, Strunk J (2017) Photocatalytic CO2 reduction under continuous flow high-purity conditions: influence of light intensity and H2O concentration. ChemCatChem 9(23):4345–4352. https://doi.org/10.1002/cctc.201701189
Zhu Z, Qin J, Jiang M, Ding Z, Hou Y (2017) Enhanced selective photocatalytic CO2 reduction into CO over Ag/CdS nanocomposites under visible light. Appl Surf Sci 391:572–579. https://doi.org/10.1016/j.apsusc.2016.06.148
Low J, Qiu S, Xu D, Jiang C, Cheng B (2018) Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl Surf Sci 434:423–432. https://doi.org/10.1016/j.apsusc.2017.10.194
Hattori Y, Abdellah M, Rocha I, Pavliuk MV, Fernandes DLA, Sá J (2018) Light-induced ultrafast proton-coupled electron transfer responsible for H2 evolution on silver plasmonics. Mater Today 21(6):590–593. https://doi.org/10.1016/j.mattod.2018.05.002
Sorcar S, Hwang Y, Grimes CA, In SI (2017) Highly enhanced and stable activity of defect-induced titania nanoparticles for solar light-driven CO2 reduction into CH4. Mater Today 20(9):507–515. https://doi.org/10.1016/j.mattod.2017.09.005
Wang T, Meng X, Liu G, Chang K, Li P, Kang Q, Liu L, Li M, Ouyang S, Ye J (2015) In situ synthesis of ordered mesoporous co-doped TiO2 and its enhanced photocatalytic activity and selectivity for the reduction of CO2. J Mater Chem A 3(18):9491–9501. https://doi.org/10.1039/C4TA05892E
Zhu W, Zhang C, Li Q, Xiong L, Chen R, Wan X, Wang Z, Chen W, Deng Z, Peng Y (2018) Selective reduction of CO2 by conductive MOF nanosheets as an efficient co-catalyst under visible light illumination. Appl Catal B: Environ 238:339–345. https://doi.org/10.1016/j.apcatb.2018.07.024
Guodong Shi LYZLXCJZYY (2018) Photocatalytic reduction of CO2 to CO over copper decorated G-C3N4 nanosheets with enhanced yield and selectivity. Appl Surf Sci 427:1165–1173. https://doi.org/10.1016/j.apsusc.2017.08.148
Zhao Y, Chen G, Bian T, Zhou C, Waterhouse GIN, Wu LZ, Tung CH, Smith LJ, O’Hare D, Zhang T (2015) Defect-rich ultrathin ZnAl-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water. Adv Mater 27(47):7824–7831. https://doi.org/10.1002/adma.201503730
Kim KH, Kim S, Moon BC, Choi JW, Jeong HM, Kwon Y, Kwon S, Choi HS, Kang JK (2017) Quadruple metal-based layered structure as the photocatalyst for conversion of carbon dioxide into a value added carbon monoxide with high selectivity and efficiency. J Mater Chem A 5(18):8274–8279. https://doi.org/10.1039/C7TA01623A
Zhao Y, Wei Y, Wu X, Zheng H, Zhao Z, Liu J, Li J (2018) Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl Catal B: Environ 226:360–372. https://doi.org/10.1016/j.apcatb.2017.12.071
Pang R, Teramura K, Tatsumi H, Asakura H, Hosokawa S, Tanaka T (2018) Modification of Ga2O3 by an Ag–Cr core–shell cocatalyst enhances photocatalytic CO evolution for the conversion of CO2 by H2O. ChemComm 54(9):1053–1056. https://doi.org/10.1039/C7CC07800E
Chen Z, Zhang G, Cao S, Chen G, Li C, Izquierdo R, Sun S (2023) Advanced semiconductor catalyst designs for the photocatalytic reduction of CO2. Materials Reports: Energy 3(2):100193. https://doi.org/10.1016/j.matre.2023.100193
Shyamal S, Pradhan N (2023) Nanostructured metal chalcohalide photocatalysts: crystal structures, synthesis, and applications. ACS Energy Lett 8(9):3902–3926. https://doi.org/10.1021/acsenergylett.3c01236
Rahman A, Khan MM (2021) Chalcogenides as photocatalysts. New J Chem 45(42):19622–19635. https://doi.org/10.1039/D1NJ04346C
Rahman A, Jennings JR, Tan AL, Khan MM (2022) Molybdenum disulfide-based nanomaterials for visible-light-induced photocatalysis. ACS Omega 7(26):22089–22110. https://doi.org/10.1021/acsomega.2c01314
Dilla M, Schlögl R, Strunk J (2017) Photocatalytic CO2 reduction under continuous flow high-purity conditions: quantitative evaluation of CH4 formation in the steady-state. ChemCatChem 9(4):696–704. https://doi.org/10.1002/cctc.201601218
El-Khouly ME, El-Mohsnawy E, Fukuzumi S (2017) Solar energy conversion: from natural to artificial photosynthesis. J Photochem Photobiol C: Photochem 31:36–83. https://doi.org/10.1016/j.jphotochemrev.2017.02.001
Ross MB, Dinh CT, Li Y, Kim D, De Luna P, Sargent EH, Yang P (2017) Tunable Cu enrichment enables designer syngas electrosynthesis from CO2. J Am Chem Soc 139(27):9359–9363. https://doi.org/10.1021/jacs.7b04892
Acknowledgements
MR gratefully acknowledges the financial support received from CSIR (EMR-II/01/3006/21), Govt of India. MR also acknowledges Summer Faculty Research Fellow Programme–2021 by IIT Delhi.
Funding
Council of Scientific and Industrial Research, India, EMR-II/01/3006/21
Author information
Authors and Affiliations
Contributions
S.G., E.G., K.C., and S.S. wrote the paper together. B.C. modified the paper. M.R. conceptualized the review, modified and communicated.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gomey, S., Guliani, E., Choudhary, K. et al. Photoactive metal chalcogenides towards CO2 reduction–a review. Colloid Polym Sci (2024). https://doi.org/10.1007/s00396-024-05235-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00396-024-05235-0