Abstract
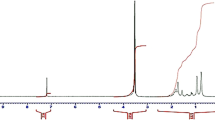
Diblock amphiphilic copolymers form aggregates in some solvents. Such aggregates exhibit different morphologies, depending mainly on the polar/apolar block ratios. Aggregation of copolymers with polar block excess leads to micelle-like aggregates, known as polymeric micelles, which can be used as vehicles for drug and gene delivery, water decontamination, and catalysis. Here, we synthesized by RAFT polymerization three different polymers namely [dimethyl 2-(aminoethyl) methacrylate] (PDMAEMA), poly (methyl methacrylate) (PMMA) copolymers, and PDMAEMA-block-PMMA and characterized their aggregates by NMR spectroscopy and pH titrations. We investigated correlations between their chemical structure, aggregation behavior, protonation degree, and chain conformation in the corona. Decreased amine protonation in the copolymers reduced the electrostatic repulsion, and the apparent pKa of the amino groups approached that of isolated amine. These effects increased compactness and sizes of the polymers and their aggregates at higher pH as reflected by the increased NMR line widths.









Similar content being viewed by others
References
Riess G (2003) Micellization of block copolymers. Prog Polym Sci 28:1107–1170
Blanazs A, Armes SP, Ryan AJ (2009) Self-assembled block copolymer aggregates: from micelles to vesicles and their biological applications. Macromol Rapid Commun 30:267–277
Nagarajan R (2015) “Non-equilibrium” block copolymer micelles with glassy cores: a predictive approach based on a theory of equilibrium micelles. J Colloid Interface Sci 449:416–427
Smart T, Lomas H, Massignani M, Flores-Merino MV, Perez LR, Battaglia G (2008) Block copolymer nanostructures. Nano Today 3:38–46
Ling P, Xu W, Zhang T (2013) Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv:1–15
Beija M, Salvayre R, Lauth-de Viguerie N, Marty JD (2012) Colloidal systems for drug delivery: from design to therapy. Trends Biotechnol 30:485–496
Guo X, Huang L (2012) Recent advances in nonviral vectors for gene delivery. Acc Chem Res 45:971–979
Huang LQ, Yuan SJ, Lv L, Tan GQ, Liang B, Pehkonen SO (2013) Poly(methacrylic acid)-grafted chitosan microspheres via surface-initiated ATRP for enhanced removal of cd(II) ions from aqueous solution. J Colloid Interface Sci 405:171–182
Ge Z, Xie D, Chen D, Jiang X, Zhang Y, Liu H, Liu S (2007) Stimuli-responsive double hydrophilic block copolymer micelles with switchable catalytic activity. Macromolecules 40:3538–3546
Braunecker WA, Matyjaszewski K (2008) Controlled/living radical polymerization: features, developments, and perspectives (vol 32, pg 93, 2007). Prog Polym Sci 33:165–165
Feng HB, Lu XY, Wang WY, Kang NG, Mays JW (2017) Block copolymers: synthesis, self-assembly, and applications. Polymers 9(10):494–525
Bettencourt A, Almeida AJ (2012) Poly(methyl methacrylate) particulate carriers in drug delivery. J Microencapsul 29:353–367
Lu Y, Park K (2013) Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm 453:198–214
Nicolai T, Colombani O, Chassenieux C (2010) Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter 6:3111–3118
Agut W, Brûlet A, Schatz C, Taton D, Lecommandoux S (2010) pH and temperature responsive polymeric micelles and polymersomes by self-assembly of poly[2-(dimethylamino)ethyl methacrylate]-b-poly(glutamic acid) double hydrophilic block copolymers. Langmuir 26:10546–10554
Dai S, Ravi P, Tam KC (2008) pH-responsive polymers: synthesis, properties, and applications. Soft Matter 4:435–449
Jochum FD, Theato P (2013) Temperature- and light-responsive smart polymer materials. Chem Soc Rev 42:7468–7483
de Souza JCP, Naves AF, Florenzano FH (2012) Specific thermoresponsiveness of PMMA-block-PDMAEMA to selected ions and other factors in aqueous solution. Colloid Polym Sci 290:1285–1291
Huang Y, Yong P, Chen Y, Gao Y, Xu W, Lv Y, Yang L, Reis RL, Pirraco RP, Chen J (2017) Micellization and gelatinization in aqueous media of pH- and thermo-responsive amphiphilic ABC (PMMA82-b-PDMAEMA150-b-PNIPAM65) triblock copolymer synthesized by consecutive RAFT polymerization. RSC Adv 7:28711–28722
Rodríguez-Hernández J, Chécot F, Gnanou Y, Lecommandoux S (2005) Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog Polym Sci 30:691–724
Baussard J-F, Habib-Jiwan J-L, Laschewsky A, Mertoglu M, Storsberg J (2004) New chain transfer agents for reversible addition-fragmentation chain transfer (RAFT) polymerization in aqueous solution. Polymer 45:3615–3626
de Souza VV, de Carvalho Noronha ML, Alves Almeida FL, Ribeiro Prado CA, Doriguetto AC, Florenzano FH (2011) CMC of PMMA-block-PDMAEMA measured by NPN fluorescence. Polym Bull 67:875–884
Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas P, Ulrich EL, Markley JL, Ionides J, Laue ED (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins Struct Funct Bioinf 59:687–696
Shen JN, Ye YF, Zeng GN, Qiu JH (2011) Preparation and characterization of PMMA-b-PDMAEMA/polysulfone composite membranes by RAFT polymerization and their permeation performance of carbon dioxide. Advanced Materials Research 284–286:1717–1723
Zhao Y, Guo K, Wang C, Wang L (2010) Effect of inclusion complexation with Cyclodextrin on the cloud point of poly(2-(dimethylamino)ethyl methacrylate) solution. Langmuir 26:8966–8970
Saitoh T, Matsushima S, Hiraide M (2004) Aerosol-OT–γ-alumina admicelles for the concentration of hydrophobic organic compounds in water. J Chromatogr A 1040:185–191
Chatterjee U, Jewrajka SK, Mandal BM (2005) The amphiphilic block copolymers of 2-(dimethylamino) ethyl methacrylate and methyl methacrylate: synthesis by atom transfer radical polymerization and solution properties. Polymer 46:10699–10708
Xiao G, Hu Z, Zeng G, Wang Y, Huang Y, Hong X, Xia B, Zhang G (2012) Effect of hydrophilic chain length on the aqueous solution behavior of block amphiphilic copolymers PMMA-b-PDMAEMA. J Appl Polym Sci 124:202–208
van de Wetering P ME, Schuurmans-Nieuwenbroek NM, van Steenbergen MJ, Hennink WE (1999) Structure-activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjug Chem 10:589–597
Israelachvili J (2011) Intermolecular and surface forces. Elsevier Inc, Amsterdam
Eastoe J (2005) Surfactant chemistry. Wuhan University Press, Wuhan
Barbieri BW, Strauss UP (1985) Effect of alkyl group size on the cooperativity in conformational transitions of hydrophobic polyacids. Macromolecules 18:411–414
Strauss UP, Barbieri BW (1982) Estimation of the cooperative unit size in conformational transitions of hydrophobic polyacids. Macromolecules 15:1347–1349
Lee H, Son SH, Sharma R, Won Y-Y (2011) A discussion of the pH-dependent protonation behaviors of poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) and poly(ethylenimine-ran-2-ethyl-2-oxazoline) (P(EI-r-EOz)). J Phys Chem B 115:844–860
Manning GS (1979) Counterion binding in polyelectrolyte theory. Acc Chem Res 12:443–449
Quina F, Chaimovich H (1979) Ion-exchange in micellar solutions .1. Conceptual-framework for ion-exchange in micellar solutions. J Phys Chem 83:1844–1850
Přádný M, Ševčík S (1987) Precursors of hydrophilic polymers, 7. Potentiometric properties and structure of copolymers of 2-dimethylaminoethyl methacrylate. Die Makromol Chem 188:227–238
Laaser JE, Jiang Y, Sprouse D, Reineke TM, Lodge TP (2015) pH- and ionic-strength-induced contraction of polybasic micelles in buffered aqueous solutions. Macromolecules 48:2677–2685
McKernan BA, Manning GS, Romsted LS (2003) Estimating concentrations of condensed counterions around a polyelectrolyte by chemical trapping. Conducting Polymers And Polymer Electrolytes: From Biology To Photovoltaics, pp. 184–199
Borukhov I, Andelman D, Borrega R, Cloitre M, Leibler L, Orland H (2000) Polyelectrolyte titration: theory and experiment. J Phys Chem B 104:11027–11034
Acknowledgments
IMC, HC, and RKS are CNPq research scholars. GKVS acknowledges the Projeto Biocomputacional/CAPES (proc. no. 23038.004630/2014-35) and CNPq (proc. 457733/2014-4).
Funding
FAPESP (grant number 2013/08166-5 and 2016/07490-1). IMC has been supported by the INCT-FCx (Instituto Nacional de Ciência e Tecnologia de Fluidos Complexos (CNPq/CAPES/FINEP/FAPESP) and NAP-FCx (Núcleo de Apoio à Pesquisa de Fluidos Complexos da Universidade de São Paulo).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 76 kb)
Rights and permissions
About this article
Cite this article
Saraiva, G.K.V., de Souza, V.V., de Oliveira, L.C. et al. Characterization of PMMA-b-PDMAEMA aggregates in aqueous solutions. Colloid Polym Sci 297, 557–569 (2019). https://doi.org/10.1007/s00396-019-04482-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04482-w