Abstract
Purpose
This study was aimed to investigate the effects of a maternal low-protein diet on transcriptional regulation of the myostatin (MSTN) gene in skeletal muscle of weaning piglets.
Methods
Sows were fed either a standard-protein (SP, 15 and 18 % crude protein) or a low-protein (LP, 50 % protein level of SP) diet throughout pregnancy and lactation. Longissimus dorsi muscle was sampled from male piglets at 28 days of age. The mRNA was determined by RT-PCR, and protein was measured by Western blot. Chromatin immunoprecipitation assay was used to determine the binding of transcription factors and histone H3 modifications on the MSTN gene promoter.
Results
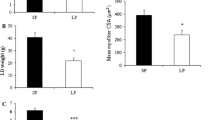
The maternal LP diet significantly decreased body weight and average daily gain (P < 0.05), which was associated with significantly lower plasma concentration of urea nitrogen and total protein (P < 0.05), as well as decreased muscle RNA content (P < 0.05). MSTN mRNA (P < 0.05) was significantly increased, together with enhanced (P < 0.05) mRNA and protein expression of forkhead box class O family member protein 3 (FoxO3), and a tendency of an increase (P = 0.10) in glucocorticoid receptor (GR) mRNA in the muscle of LP piglets. Furthermore, the binding of both FoxO3 and GR to the MSTN gene promoter was significantly higher (P < 0.05) in muscle of LP piglets, together with significantly enriched (P < 0.05) gene activation markers, H3K9Ac and H3K4me3.
Conclusion
These results indicate that MSTN mediates maternal LP diet-induced growth retardation, through epigenetic regulation involving FoxO3 and GR binding to its promoter.



Similar content being viewed by others
Abbreviations
- ChIP:
-
Chromatin immunoprecipitation
- FoxO3:
-
Forkhead box class O family member protein 3
- GR:
-
Glucocorticoid receptor
- LM:
-
Longissimus dorsi muscle
- LP:
-
Low protein
- MSTN:
-
Myostatin
- SP:
-
Standard protein
References
Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G (2003) Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286:263–275
Welle S, Burgess K, Thornton CA, Tawil R (2009) Relation between extent of myostatin depletion and muscle growth in mature mice. Am J Physiol Endocrinol Metab 297:E935–E940
Wang Q, McPherron AC (2012) Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J Physiol 590:2151–2165
Hennebry A, Berry C, Siriett V, O’Callaghan P, Chau L, Watson T, Sharma M, Kambadur R (2009) Myostatin regulates fiber-type composition of skeletal muscle by regulating MEF2 and MyoD gene expression. Am J Physiol Cell Physiol 296:C525–C534
Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF (2003) Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab 285:E876–E888
Zhu X, Topouzis S, Liang LF, Stotish RL (2004) Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 26:262–272
Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D (2006) Extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res 66:1320–1326
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Peiris HN, Ponnampalam AP, Osepchook CC, Mitchell MD, Green MP (2010) Placental expression of myostatin and follistatin-like-3 protein in a model of developmental programming. Am J Physiol Endocrinol Metab 298:E854–E861
Reed SA, Raja JS, Hoffman ML, Zinn SA, Govoni KE (2014) Poor maternal nutrition inhibits muscle development in ovine offspring. J Anim Sci Biotechnol 5(1):43
Liu X, Wang J, Li R, Yang X, Sun Q, Albrecht E, Zhao R (2011) Maternal dietary protein affects transcriptional regulation of myostatin gene distinctively at weaning and finishing stages in skeletal muscle of Meishan pigs. Epigenetics 6:899–907
McMillen IC, Robinson JS (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633
Jones PA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13:484–492
Shahbazian MD, Grunstein M (2007) Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76:75–100
Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD (2010) Histone methylation regulates memory formation. J Neurosci 30:3589–3599
Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, Casero RA Jr (2007) Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proc Natl Acad Sci USA 104:8023–8028
Bettini ML, Pan F, Bettini M, Finkelstein D, Rehg JE, Floess S, Bell BD, Ziegler SF, Huehn J, Pardoll DM, Vignali DA (2012) Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity 36:717–730
Watson ML, Baehr LM, Reichardt HM, Tuckermann JP, Bodine SC, Furlow JD (2012) A cell-autonomous role for the glucocorticoid receptor in skeletal muscle atrophy induced by systemic glucocorticoid exposure. Am J Physiol Endocrinol Metab 302:E1210–E1220
Allen DL, Unterman TG (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292:C188–C199
Johnson LR, Chandler AM (1973) RNA and DNA of gastric and duodenal mucosa in antrectomized and gastrin-treated rats. Am J Physio 224:937–940
Dwyer CM, Stickland NC, Fletcher JM (1994) The influence of maternal nutrition on muscle fiber number development in the porcine fetus and on subsequent postnatal growth. J Anim Sci 72:911–917
Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW (2006) A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571:221–230
Addis T, Barrett E, Poo LJ, Yuen DW (1947) The relation between the serum urea concentration and the protein consumption of normal individuals. J Clin Invest 26:869–874
Wu G, Pond WG, Ott T, Bazer FW (1998) Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr 128:894–902
Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS (1973) Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241:204–205
Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45:2163–2172
Ma K, Mallidis C, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S (2001) Characterization of 5′-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab 281:E1128–E1136
Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B (2003) Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab 285:E363–E371
Du R, An XR, Chen YF, Qin J (2007) Some motifs were important for myostatin transcriptional regulation in sheep (Ovis aries). J Biochem Mol Biol 40:547–553
Qin J, Du R, Yang YQ, Zhang HQ, Li Q, Liu L, Guan H, Hou J, An XR (2013) Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res Vet Sci 94:84–89
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6:458–471
Han DS, Huang HP, Wang TG, Hung MY, Ke JY, Chang KT, Chang HY, Ho YP, Hsieh WY, Yang WS (2010) Transcription activation of myostatin by trichostatin A in differentiated C2C12 myocytes via ASK1-MKK3/4/6-JNK and p38 mitogen-activated protein kinase pathways. J Cell Biochem 111:564–573
Acknowledgments
We thank Shanghai Farm of Bright Food (Group) Co., Ltd., for providing the experimental site and Rongkui Zhang for care of animals. In addition, we thank Dr. Wanyne J. Kuenzel of University of Arkansas for his suggestions on paper writing, especially for language revision. This work was supported by the National Basic Research Program of China (2012CB124703), the Special Fund for Agro-scientific Research in the Public Interest (201003011), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of interest
Yimin Jia, Guichao Gao, Haogang Song, Demin Cai, Xiaojing Yang, and Ruqian Zhao declare no conflicts of interest.
Ethical standards
Operating procedures for animal breeding and husbandry were in accordance with Technical Regulations for Commercial Pig Production for Intensive Pig Farms (GB/T 17824.2-2008). The experimental protocol was approved by the Animal Ethics Committee of Nanjing Agricultural University with the project number 2012CB124703. The slaughter and sampling procedures complied with the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 set by the Ministry of Science and Technology, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, Y., Gao, G., Song, H. et al. Low-protein diet fed to crossbred sows during pregnancy and lactation enhances myostatin gene expression through epigenetic regulation in skeletal muscle of weaning piglets. Eur J Nutr 55, 1307–1314 (2016). https://doi.org/10.1007/s00394-015-0949-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0949-3