Abstract
Purpose
The contribution of extra virgin olive oil (EVOO) macro- and micro-constituents in heart oxidative and inflammatory status in a hypercholesterolemic rat model was evaluated. Fatty acid profile as well as α-tocopherol, sterol, and squalene content was identified directly in rat hearts to distinguish the effect of individual components or to enlighten the potential synergisms.
Methods
Oils and oil-products with discernible lipid and polar phenolic content were used. Wistar rats were fed a high-cholesterol diet solely, or supplemented with one of the following oils, i.e., EVOO, sunflower oil (SO), and high-oleic sunflower oil (HOSO) or oil-products, i.e., phenolics-deprived EVOO [EVOO(−)], SO enriched with the EVOO phenolics [SO(+)], and HOSO enriched with the EVOO phenolics [HOSO(+)]. Dietary treatment lasted 9 weeks; at the end of the intervention blood and heart samples were collected.
Results
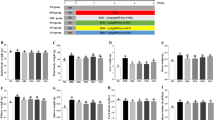
High-cholesterol-diet-induced dyslipidemia was shown by increase in serum total cholesterol, low-density lipoprotein cholesterol, and triacylglycerols. Dyslipidemia resulted in increased malondialdehyde (MDA) and tumor necrosis factor-α (TNF-α) levels, while glutathione and interleukin 6 levels remained unaffected in all intervention groups. Augmentation observed in MDA and TNF-α was attenuated in EVOO, SO(+), and HOSO(+) groups. Heart squalene and cholesterol content remained unaffected among all groups studied. Heart α-tocopherol was determined by oil α-tocopherol content. Variations were observed for heart β-sitosterol, while heterogeneity was reported with respect to heart fatty acid profile in all intervention groups.
Conclusions
Overall, we suggest that the EVOO-polar phenolic compounds decreased MDA and TNF-α in hearts of cholesterol-fed rats.
Similar content being viewed by others
References
Go AS, Mozaffarian D, Roger VL et al (2014) Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129(3):e28–e292. doi:10.1161/01.cir.0000441139.02102.80
Covas MI (2008) Bioactive effects of olive oil phenolic compounds in humans: reduction of heart disease factors and oxidative damage. Inflammopharmacology 16(5):216–218. doi:10.1007/s10787-008-8019-6
Jiangwei M, Zengyong Q, Xia X (2011) Aqueous extract of Astragalus mongholicus ameliorates high cholesterol diet induced oxidative injury in experimental rats models. J Med Plants Res 5(5):855–858
Ozden S, Alpertunga B (2010) Effects of methiocarb on lipid peroxidation and glutathione level in rat tissues. Drug Chem Toxicol 33(1):50–54. doi:10.3109/01480540903130708
Al-Shudiefat AA, Sharma AK, Bagchi AK et al (2013) Oleic acid mitigates TNF-alpha-induced oxidative stress in rat cardiomyocytes. Mol Cell Biochem 372(1–2):75–82. doi:10.1007/s11010-012-1447-z
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16):1685–1695. doi:10.1056/NEJMra043430
Ridker PM, Rifai N, Pfeffer M et al (2000) Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101(18):2149–2153
Ma Z, Zhang J, Du R et al (2011) Rho kinase inhibition by fasudil has anti-inflammatory effects in hypercholesterolemic rats. Biol Pharm Bull 34(11):1684–1689
Mente A, de Koning L, Shannon HS et al (2009) A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 169(7):659–669. doi:10.1001/archinternmed.2009.38
Covas MI, Konstantinidou V, Fito M (2009) Olive oil and cardiovascular health. J Cardiovasc Pharmacol 54(6):477–482. doi:10.1097/FJC.0b013e3181c5e7fd
Schwingshackl L, Hoffmann G (2014) Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis 13:154. doi:10.1186/1476-511X-13-154
Covas MI, Ruiz-Gutierrez V, De la Torre R et al. (2006) Minor components of olive oil: evidence to date of health benefits in humans. Nutr Rev 64(10):S20–S30. doi:10.1301/nr.2006.oct.S20–S30
Vivancos M, Moreno JJ (2008) Effect of resveratrol, tyrosol and beta-sitosterol on oxidised low-density lipoprotein-stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by RAW 264.7 macrophages. Br J Nutr 99(6):1199–1207. doi:10.1017/S0007114507876203
Saeidnia S, Manayi A, Gohari AR et al (2014) The story of beta-sitosterol—a review. Eur J Med Plants 4(5):590–609
Xu J, Zhou X, Deng Q et al (2011) Rapeseed oil fortified with micronutrients reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis 10:96. doi:10.1186/1476-511X-10-96
Karantonis HC, Zabetakis I, Nomikos T et al (2003) Antiatherogenic properties of lipid minor constituents from seed oils. J Sci Food Agric 83(12):1192–1204
Deiana M, Rosa A, Corona G et al (2007) Protective effect of olive oil minor polar components against oxidative damage in rats treated with ferric-nitrilotriacetate. Food Chem Toxicol 45(12):2434–2440. doi:10.1016/j.fct.2007.06.028
Montero MF, Saurim R, Bonservizi WG et al (2014) Heart injury following intestinal ischemia reperfusion in rats is attenuated by association of ischemic preconditioning and adenosine. Acta Cir Bras 29(Suppl 2):67–71
Gioxari A, Kaliora AC, Papalois A et al (2011) Pistacia lentiscus resin regulates intestinal damage and inflammation in trinitrobenzene sulfonic acid-induced colitis. J Med Food 14(11):1403–1411. doi:10.1089/jmf.2010.0240
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Iverson SJ, Lang SL, Cooper MH (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36(11):1283–1287
Gutfinger T (1981) Polyphenols in olive oils. J Am Chem Soc 58(11):966–968
Anastasopoulos E, Kalogeropoulos N, Kaliora AC et al (2012) Quality characteristics and antioxidants of mavrolia cv. virgin olive oil. J Am Oil Chem Soc 89(2):253–259
Chiou A, Kalogeropoulos N, Efstathiou P et al (2013) French Fries oleuropein content during the successive deep frying in oils enriched with an olive leaf extract. Int J Food Sci Technol 48(6):1165–1171. doi:10.1111/ijfs.12070
Chiou A, Kalogeropoulos N, Salta FN et al (2009) Pan-frying of French fries in three different edible oils enriched with olive leaf extract: oxidative stability and fate of microconstituents. LWT Food Sci Technol 42(6):1090–1097
Boskou D (2008) Phenolic compounds in olives and olive oil. In: Boskou D (ed) Olive oil: minor constituents and health. CRC Press, Boca Raton, pp 11–44
Di Benedetto R, Attorri L, Chiarotti F et al (2010) Effect of micronutrient-enriched sunflower oils on plasma lipid profile and antioxidant status in high-fat-fed rats. J Agric Food Chem 58(9):5328–5333. doi:10.1021/jf904360y
Gorinstein S, Leontowicz H, Leontowicz M et al (2003) Seed oils improve lipid metabolism and increase antioxidant potential in rats fed diets containing cholesterol. Nutr Res 23(3):317–330
Radcliffe JD, Czajka-Narins DM (2006) Lipids and tocopherols in serum and liver of female rats fed diets containing corn oil or cottonseed oil. Plant Foods Hum Nutr 61(1):35–38. doi:10.1007/s11130-006-0011-y
Chan YM, Demonty I, Pelled D et al (2007) Olive oil containing olive oil fatty acid esters of plant sterols and dietary diacylglycerol reduces low-density lipoprotein cholesterol and decreases the tendency for peroxidation in hypercholesterolaemic subjects. Br J Nutr 98(3):563–570. doi:10.1017/S0007114507730775
Chetty KN, Calahan L, Harris KC et al (2003) Garlic attenuates hypercholesterolemic risk factors in olive oil fed rats and high cholesterol fed rats. Pathophysiology 9(3):127–132
Calandra S, Pasquali-Ronchetti I, Gherardi E et al (1977) Chemical and morphological changes of rat plasma lipoproteins after a prolonged administration of diets containing olive oil and cholesterol. Atherosclerosis 28(4):369–387
Joris I, Zand T, Nunnari JJ et al (1983) Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol 113(3):341–358
Takeuchi H, Nakamoto T, Mori Y et al (2001) Comparative effects of dietary fat types on hepatic enzyme activities related to the synthesis and oxidation of fatty acid and to lipogenesis in rats. Biosci Biotechnol Biochem 65(8):1748–1754
Ferramosca A, Savy V, Zara V (2008) Olive oil increases the hepatic triacylglycerol content in mice by a distinct influence on the synthesis and oxidation of fatty acids. Biosci Biotechnol Biochem 72(1):62–69
Jemai H, Bouaziz M, Fki I et al (2008) Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem Biol Interact 176(2–3):88–98. doi:10.1016/j.cbi.2008.08.014
Tabernero M, Sarria B, Largo C et al (2014) Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food Funct 5(7):1556–1563. doi:10.1039/c3fo60677e
Fki I, Bouaziz M, Sahnoun Z et al (2005) Hypocholesterolemic effects of phenolic-rich extracts of Chemlali olive cultivar in rats fed a cholesterol-rich diet. Bioorg Med Chem 13(18):5362–5370. doi:10.1016/j.bmc.2005.05.036
Moghadasian MH (2002) Experimental atherosclerosis: a historical overview. Life Sci 70(8):855–865
Nielsen NS, Pedersen A, Sandstrom B et al (2002) Different effects of diets rich in olive oil, rapeseed oil and sunflower-seed oil on postprandial lipid and lipoprotein concentrations and on lipoprotein oxidation susceptibility. Br J Nutr 87(5):489–499. doi:10.1079/BJNBJN2002567
Ochoa JJ, Quiles JL, Ibanez S et al (2003) Aging-related oxidative stress depends on dietary lipid source in rat postmitotic tissues. J Bioenerg Biomembr 35(3):267–275
Bayram B, Ozcelik B, Grimm S et al (2012) A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res 15(1):71–81. doi:10.1089/rej.2011.1245
Ghosh S, Kewalramani G, Yuen G et al (2006) Induction of mitochondrial nitrative damage and cardiac dysfunction by chronic provision of dietary omega-6 polyunsaturated fatty acids. Free Radic Biol Med 41(9):1413–1424. doi:10.1016/j.freeradbiomed.2006.07.021
Paul S, Mohanan A, Varghese MV et al (2012) Ameliorative effect of alpha-tocopherol on monosodium glutamate-induced cardiac histological alterations and oxidative stress. J Sci Food Agric 92(15):3002–3006. doi:10.1002/jsfa.5714
Ciftci O, Disli OM, Timurkaan N (2013) Protective effects of protocatechuic acid on TCDD-induced oxidative and histopathological damage in the heart tissue of rats. Toxicol Ind Health 29(9):806–811. doi:10.1177/0748233712442735
Molinar-Toribio E, Perez-Jimenez J, Ramos-Romero S et al (2014) Cardiovascular disease-related parameters and oxidative stress in SHROB rats, a model for metabolic syndrome. PLoS ONE 9(8):e104637. doi:10.1371/journal.pone.0104637
Olorunnisola OS, Bradley G, Afolayan AJ (2012) Protective effect of T. violacea rhizome extract against hypercholesterolemia-induced oxidative stress in wistar rats. Molecules 17(5):6033–6045. doi:10.3390/molecules17056033
Abe C, Ikeda S, Uchida T et al (2007) Triton WR1339, an inhibitor of lipoprotein lipase, decreases vitamin E concentration in some tissues of rats by inhibiting its transport to liver. J Nutr 137(2):345–350
Jenkins MY, Mitchell GV, Grundel E (2000) Natural tocopherols in a dietary supplement of lutein affect tissue distribution of tocopherols in young rats. Nutr Cancer 37(2):207–214. doi:10.1207/S15327914NC372_14
Elmarakby AA, Sullivan JC (2012) Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther 30(1):49–59. doi:10.1111/j.1755-5922.2010.00218.x
Jain SK, Rains J, Croad J et al (2009) Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal 11(2):241–249. doi:10.1089/ars.2008.2140
Balkova P, Jezkova J, Hlavackova M et al (2009) Dietary polyunsaturated fatty acids and adaptation to chronic hypoxia alter acyl composition of serum and heart lipids. Br J Nutr 102(9):1297–1307. doi:10.1017/S0007114509389242
Nikolaidis MG, Petridou A, Mougios V (2006) Comparison of the phospholipid and triacylglycerol fatty acid profile of rat serum, skeletal muscle and heart. Physiol Res 55(3):259–265
Kalogeropoulos N, Panagiotakos DB, Pitsavos C et al (2010) Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta 411(7–8):584–591. doi:10.1016/j.cca.2010.01.023
Farvin KH, Anandan R, Kumar SH et al (2006) Cardioprotective effect of squalene on lipid profile in isoprenaline-induced myocardial infarction in rats. J Med Food 9(4):531–536. doi:10.1089/jmf.2006.9.531
Vivancos M, Moreno JJ (2005) Beta-sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic Biol Med 39(1):91–97. doi:10.1016/j.freeradbiomed.2005.02.025
Paniagua-Perez R, Madrigal-Bujaidar E, Reyes-Cadena S et al (2008) Cell protection induced by beta-sitosterol: inhibition of genotoxic damage, stimulation of lymphocyte production, and determination of its antioxidant capacity. Arch Toxicol 82(9):615–622. doi:10.1007/s00204-007-0277-3
Rubis B, Paszel A, Kaczmarek M et al (2008) Beneficial or harmful influence of phytosterols on human cells? Br J Nutr 100(6):1183–1191. doi:10.1017/S0007114508981423
Danesi F, Ferioli F, Caboni MF et al (2011) Phytosterol supplementation reduces metabolic activity and slows cell growth in cultured rat cardiomyocytes. Br J Nutr 106(4):540–548. doi:10.1017/S0007114511000626
Acknowledgments
This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) Research Funding Program “Heraclitus II—Investing in knowledge society through the European Social Fund”. This work was funded by Grant by the Experimental Research Center of ELPEN S.A. Pharmaceuticals, Pikermi, Athens, Greece (E.R.C.E.). Professor V. T. Karathanos is gratefully acknowledged for his valuable comments and fruitful conversations. We are grateful to Mr. Kyriopoulos K. (MINERVA S.A., Athens, Greece) for his help with olive oil supplementation.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsarou, A.I., Kaliora, A.C., Chiou, A. et al. Amelioration of oxidative and inflammatory status in hearts of cholesterol-fed rats supplemented with oils or oil-products with extra virgin olive oil components. Eur J Nutr 55, 1283–1296 (2016). https://doi.org/10.1007/s00394-015-0947-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0947-5