Abstract
Purpose
Enhanced recovery after surgery (ERAS) protocols have been implemented across a variety of disciplines to improve outcomes. Herein we describe the impact of ERAS on quality of life (QOL) and cost for patients undergoing urologic oncology surgery.
Methods
A systematic literature search using the MEDLINE, Scopus, Clinictrials.gov, and Cochrane Review databases for studies published between 1946 and 2020 was conducted. Articles were reviewed and assigned a risk of bias by two authors and were included if they addressed ERAS and either QOL or cost-effectiveness for patients undergoing urologic oncology surgery.
Results
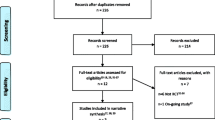
The literature search yielded a total of 682 studies after removing duplicates, of which 10 (1.5%) were included in the review. Nine articles addressed radical cystectomy, while one addressed ERAS and QOL for laparoscopic nephrectomy. Six publications assessed the impact of ERAS on QOL domains. Questionnaires used for assessment of QOL varied across studies, and timing of administration was heterogeneous. Overall, ERAS improved patient QOL during early phases of recovery within the realms of bowel function, physical/social/cognitive functioning, sleep and pain control. Costs were assessed in 4 retrospective studies including 3 conducted in the United States and one from China all addressing radical cystectomy. Studies demonstrated either decreased costs associated with ERAS as a result of decreased length of stay or no change in cost based on ERAS implementation.
Conclusion
While limited studies are published on the subject, ERAS implementation for radical cystectomy and laparoscopic nephrectomy improved patient-reported QOL during early phases of recovery. For radical cystectomy, there was a decreased or neutral overall financial cost associated with ERAS. Further studies assessing QOL and cost-effectiveness over the entire global period of care in a variety of urologic oncology surgeries are warranted.

Similar content being viewed by others
References
Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78:606–617
Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M et al (2013) Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery ERAS society recommendations. Clin Nutr 32:879–887
Williams SB, Cumberbatch MG, Kamat AM, Jubber I, Kerr PS, McGrath J, et al. Reporting Radical Cystectomy Outcomes Following Implementation of Enhanced Recovery after Surgery (ERAS) Protocols: A Systematic Review and Individual Patient Data Meta-analysis European Urology. 2020; In Press
Patel HRH, Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O et al (2014) Enhanced Recovery After Surgery: are we ready, and can we afford not to implement these pathways for patients undergoing radical cystectomy? Eur Urol 65:263–266
Moschini M, Simone G, Stenzl A, Gill IS, Catto J (2016) Critical review of outcomes from radical cystectomy: can complications from radical cystectomy be reduced by surgical volume and robotic surgery? Eur Urol Focus. 2:19–29
Tyson MD, Chang SS (2016) Enhanced recovery pathways versus standard care after cystectomy: a meta-analysis of the effect on perioperative outcomes. Eur Urol 70:995–1003
Porter ME (2009) A strategy for health care reform–toward a value-based system. N Engl J Med 361:109–112
Elias KM, Stone AB, McGinigle K, Tankou JAI, Scott MJ, Fawcett WJ et al (2019) The reporting on ERAS compliance, outcomes, and elements research (RECOvER) checklist: a joint statement by the ERAS® and ERAS® USA societies. World J Surg 43:1–8
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377
Trac MH, McArthur E, Jandoc R, Dixon SN, Nash DM, Hackam DG et al (2016) Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. Can Med Assoc J 188:E120
Frees SK, Aning J, Black P, Struss W, Bell R, Chavez-Munoz C et al (2018) A prospective randomized pilot study evaluating an ERAS protocol versus a standard protocol for patients treated with radical cystectomy and urinary diversion for bladder cancer. World J Urol 36:215–220
Karl A, Buchner A, Becker A, Staehler M, Seitz M, Khoder W et al (2014) A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: results of a Prospective Randomized Study. J Urol. 191:335–340
Jensen BT, Jensen JB, Laustsen S, Petersen AK, Søndergaard I, Borre M (2014) Multidisciplinary rehabilitation can impact on health-related quality of life outcome in radical cystectomy: secondary reported outcome of a randomized controlled trial. J Multidiscip Healthc. 7:301–311
Recart A, Duchene D, White PF, Thomas T, Johnson DB, Cadeddu JA (2005) Efficacy and safety of fast-track recovery strategy for patients undergoing laparoscopic nephrectomy. J Endourol 19:1165–1169
Kukreja JB, Shi Q, Chang CM, Seif MA, Sterling BM, Chen T-Y et al (2018) Patient-reported outcomes are associated with enhanced recovery status in patients with bladder cancer undergoing radical cystectomy. Surg Innovation. 25:242–250
Zhang H, Wang H, Zhu M, Xu Z, Shen Y, Zhu Y et al (2020) Implementation of enhanced recovery after surgery in patients undergoing radical cystectomy: a retrospective cohort study. Eur J Surg Oncol 46:202–208
Chipollini J, Tang DH, Hussein K, Patel SY, Garcia-Getting RE, Pow-Sang JM et al (2017) Does implementing an enhanced recovery after surgery protocol increase hospital charges? comparisons from a radical cystectomy program at a specialty cancer center. Urology. 105:108–112
Nabhani J, Ahmadi H, Schuckman AK, Cai J, Miranda G, Djaladat H et al (2016) Cost analysis of the enhanced recovery after surgery protocol in patients undergoing radical cystectomy for bladder cancer. Eur Urol Focus. 2:92–96
Wei C, Wan F, Zhao H, Ma J, Gao Z, Lin C (2018) Application of enhanced recovery after surgery in patients undergoing radical cystectomy. J Int Med Res 46:5011–5018
Semerjian A, Milbar N, Kates M, Gorin MA, Patel HD, Chalfin HJ et al (2018) Hospital charges and length of stay following radical cystectomy in the enhanced recovery after surgery era. Urology. 111:86–91
Myles PS, Weitkamp B, Jones K, Melick J, Hensen S (2000) Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 84:11–15
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI J Natl Cancer Inst. 85:365–376
Brédart A, Bottomley A, Blazeby JM, Conroy T, Coens C, D’Haese S et al (2005) An international prospective study of the EORTC cancer in-patient satisfaction with care measure (EORTC IN-PATSAT32). Eur J Cancer 41:2120–2131
Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG (2000) Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 56:899–905
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Botteman MF, Pashos CL, Hauser RS, Laskin BL, Redaelli A (2003) Quality of life aspects of bladder cancer: a review of the literature. Quality Life Res 12:675–688
Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M et al (2000) Assessing symptom distress in cancer patients. Cancer 89:1634–1646
Dominique I, Palamara C, Champetier D, Terrier JE, Morel Journel N, Ruffion A et al (2019) Réhabilitation précoce après tumorectomie rénale robot-assistée: quelle satisfaction des patients ? Progrès en Urologie. 29:634–641
Mason SJ, Downing A, Wright P, Hounsome L, Bottomley SE, Corner J et al (2018) Health-related quality of life after treatment for bladder cancer in England. Br J Cancer 118:1518–1528
Mason SJ, Catto JWF, Downing A, Bottomley SE, Glaser AW, Wright P (2018) Evaluating patient-reported outcome measures (PROMs) for bladder cancer: a systematic review using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist. BJU Int. 122:760–773
Catto JWF, Khetrapal P, Ambler G, Sarpong R, Potyka I, Khan MS et al (2018) Multidomain quantitative recovery following radical cystectomy for patients within the robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy randomised controlled trial: the first 30 patients. Eur Urol 74:531–534
Ziegelmueller BK, Jokisch JF, Buchner A, Grimm T, Kretschmer A, Schulz GB, Stief C, Karl A (2020) Long-term follow-up and oncological outcome of patients undergoing radical cystectomy for bladder cancer following an Enhanced Recovery after Surgery (ERAS) protocol: results of a large randomized, prospective. Single-Center Study. Urologia Internationalis. 104(1–2):54–60
Eskicioglu C, Forbes SS, Aarts M-A, Okrainec A, McLeod RS (2009) Enhanced Recovery after Surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointestinal Surg. 13:2321
Li D, Jensen CC (2019) Patient satisfaction and quality of life with enhanced recovery protocols. Clin Colon Rectal Surg 32:138–144
Day RW, Cleeland CS, Wang XS, Fielder S, Calhoun J, Conrad C et al (2015) Patient-reported outcomes accurately measure the value of an enhanced recovery program in liver surgery. J Am Coll Surg 221(1023–30):e2
Jones E, Wainwright T, Foster J, Smith J, Middleton R, Francis N (2014) A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl. 96:89–94
Rauwerdink A, Jansen M, De Borgie C, Bemelman W, Daams F, Schijven M et al (2019) Improving enhanced recovery after surgery (ERAS): eRAS APPtimize study protocol, a randomized controlled trial investigating the effect of a patient-centred mobile application on patient participation in colorectal surgery. BMC Surg. 19:125
Zheng Y, Mao M, Ji M, Zheng Q, Liu L, Zhao Z et al (2020) Does a pulmonary rehabilitation based ERAS program (PREP) affect pulmonary complication incidence, pulmonary function and quality of life after lung cancer surgery? study protocol for a multicenter randomized controlled trial. BMC Pulmonary Med. 20:44
Manger JP, Nelson M, Blanchard S, Helo S, Conaway M, Krupski TL (2014) Alvimopan: a cost-effective tool to decrease cystectomy length of stay. Cent Eur J Urol. 67:335–341
Ghodoussipour S, Naser-Tavakolian A, Cameron B, Mitra AP, Miranda G, Cai J et al (2020) Internal audit of an enhanced recovery after surgery protocol for radical cystectomy. World J Urol. https://doi.org/10.1007/s00345-020-03135-w
Joliat G-R, Ljungqvist O, Wasylak T, Peters O, Demartines N (2018) Beyond surgery: clinical and economic impact of Enhanced Recovery After Surgery programs. BMC Health Services Res. 18:1008
Gentry ZL, Boitano TK, Smith HJ, Eads DK, Russell JF, Straughn JM Jr (2020) The financial impact of an enhanced recovery after surgery (ERAS) protocol in an academic gynecologic oncology practice. Gynecol Oncol 156:284–287
Lee L, Mata J, Ghitulescu GA, Boutros M, Charlebois P, Stein B et al (2015) Cost-effectiveness of Enhanced Recovery Versus Conventional Perioperative Management for Colorectal Surgery. Ann Surg 262:1026–1033
Abola RE, Bennett-Guerrero E, Kent ML, Feldman LS, Fiore JFJ, Shaw AD et al (2018) American Society for enhanced recovery and perioperative quality initiative joint consensus statement on patient-reported outcomes in an enhanced recovery pathway. Anesth Analg 126:1874–1882
Acknowledgements
This study was conducted with the support of a Department of Defense Peer Reviewed Cancer Research Program (PRCRP) Career Development Award (W81XWH1710576) (SBW). Dr. Stephen B. Williams had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We would also like to thank Julie M. Trumble and Stephen Schuenke for their efforts in preparing this manuscript for publication.
Author information
Authors and Affiliations
Contributions
NAB—protocol/project development, data collection or management, data analysis, manuscript writing/editing. AK—protocol/project development, data collection or management, data analysis, manuscript writing/editing. JMG—manuscript writing/editing. WK—manuscript writing/editing. JWC—manuscript writing/editing. PCB—manuscript writing/editing. JD—manuscript writing/editing. HD—manuscript writing/editing. SD—manuscript writing/editing. JWFC—manuscript writing/editing. AMK—manuscript writing/editing. SBW—protocol/project development, data collection or management, data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
JWFC has received reimbursement for consultancy from Astra Zeneca, Roche and Janssen, speaker fees from BMS, MSD, Nucleix and Roche, and honoraria for membership of advisory boards for Ferring, Roche and Janssen; SBW has received reimbursement for consultancy from Photocure and Taris. SBW has received travel funding from Janssen. The remaining authors have no conflicts of interest to declare.
Research involving Human Participants and/or Animals
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1.
PRISMA Checklist.
Rights and permissions
About this article
Cite this article
Brooks, N.A., Kokorovic, A., McGrath, J.S. et al. Critical analysis of quality of life and cost-effectiveness of enhanced recovery after surgery (ERAS) for patient’s undergoing urologic oncology surgery: a systematic review. World J Urol 40, 1325–1342 (2022). https://doi.org/10.1007/s00345-020-03341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-020-03341-6