Abstract
Objectives
To generate and validate a prediction model based on imaging features for cancer risk of non-mass lesions (NMLs) detected on breast ultrasound (US).
Methods
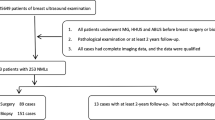
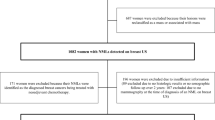
In this single-center study, consecutive women with 503 NMLs detected on breast US between 2012 and 2019 were retrospectively identified. The lesions were randomly assigned to the training or testing dataset with a 70/30 split. Age, symptoms, lesion size, and US features were collected. Multivariate analyses were employed to identify risk factors associated with malignancy. The predictive model was developed by using conditional inference trees (CTREE).
Results
There were 498 patients (50.9 ± 13.29 years; range, 22–88 years) with 503 NMLs with histopathologic results or > 2-year follow-up, including 224 (44.5%) benign and 279 (55.5%) malignant lesions. At multivariate analysis, age (odds ratio (OR) = 1.08, 95% confidence interval (CI), 1.06–1.11, p < 0.001), NMLs with focal mass effect (OR = 3.03, 95% CI, 1.59–5.81, p = 0.001), indistinct glandular–fat interface (GFI) (OR = 4.23, 95% CI, 2.31–7.73, p < 0.001), geographic (OR = 3.47, 95% CI, 1.20–10.8, p = 0.022) and mottled (OR = 3.67, 95% CI, 1.32–10.21, p = 0.013) patterns, and calcifications (OR = 2.15, 95% CI, 1.16–4.01, p = 0.016) were associated with malignancy. The GFI status, architectural patterns, general morphology, and calcifications were consistently identified as the strongest US predictors of malignancy using CTREE analysis. Based on these factors, individuals were stratified into six risk groups. The predictive model showed an area under the curve of 0.797 in the testing dataset.
Conclusion
The CTREE model efficiently aids in interpreting and managing ultrasound-detected breast NMLs, overcoming BI-RADS limitations by refining cancer risk stratification.
Clinical relevance statement
The CTREE model allows for the reclassification of BI-RADS categories into subgroups with varying malignancy probabilities, thus providing a valuable enhancement to the BI-RADS assessment for the diagnosis of ultrasound-detected NMLs, with the potential to minimize unnecessary biopsies.
Key Points
• The indistinct glandular–fat interface (GFI) status, NML with focal mass effect, geographic or mottled patterns, and calcifications are the strongest imaging predictors of malignant non-mass lesions (NMLs) detected on breast US.
• A practical system has been created to categorize NMLs found in breast US; each classification is associated with a degree of diagnostic certainty.
• The model may contribute to patient stratification by determining the relative likelihood of malignancy and thus support clinical decision-making and evidence-based management.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- BI-RADS:
-
Breast Imaging Reporting and Data System
- CI:
-
Confidence interval
- DCIS:
-
Ductal carcinoma in situ
- GFI:
-
Glandular–fat interface
- NML:
-
Non-mass lesion
- OR:
-
Odds ratio
- US:
-
Ultrasonography
References
Ohuchi N, Suzuki A, Sobue T et al (2016) Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet 387(10016):341–348
Pan H-B (2016) The Role of Breast Ultrasound in Early Cancer Detection. J Med Ultrasound 24(4):138–141
Kim SJ, Park YM, Jung HK (2014) Nonmasslike lesions on breast sonography: comparison between benign and malignant lesions. J Ultrasound Med 33(3):421–430
Lee J, Lee JH, Baik S et al (2016) Non-mass lesions on screening breast ultrasound. Med Ultrason 18(4):446–451
Shin HJ, Kim HH, Kim SM, Kwon GY, Gong G, Cho OK (2008) Screening-detected and symptomatic ductal carcinoma in situ: differences in the sonographic and pathologic features. AJR Am J Roentgenol 190(2):516–525
Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH (2014) Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol 24(2):305–311
Ko KH, Hsu HH, Yu JC et al (2015) Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol 84(1):77–85
EBM, MB-V, WAB (2013) ACR BI-RADS® Ultrasound. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology, Reston, VA
Choe J, Chikarmane SA, Giess CS (2020) Nonmass Findings at Breast US: Definition, Classifications, and Differential Diagnosis. Radiographics 40(2):326–335
Tot T (2007) Clinical relevance of the distribution of the lesions in 500 consecutive breast cancer cases documented in large-format histologic sections. Cancer 110(11):2551–2560
Tot T (2011) Subgross morphology, the sick lobe hypothesis, and the success of breast conservation. Int J Breast Cancer. https://doi.org/10.4061/2011/634021
Chadashvili T, Ghosh E, Fein-Zachary V et al (2015) Nonmass enhancement on breast MRI: review of patterns with radiologic-pathologic correlation and discussion of management. AJR Am J Roentgenol 204(1):219–227
Weaver O, Yang W (2020) Imaging of Breast Cancers With Predilection for Nonmass Pattern of Growth: Invasive Lobular Carcinoma and DCIS-Does Imaging Capture It All? AJR Am J Roentgenol 215(6):1504–1511
Uematsu T (2012) Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer 19(4):295–301
Li LJ, Zhou XC, Zhao XB et al (2017) B-Mode Ultrasound Combined with Color Doppler and Strain Elastography in the Diagnosis of Non-mass Breast Lesions: A Prospective Study. Ultrasound Med Biol 43(11):2582–2590
Zhang WY, Xiao XY, Xu XL et al (2018) Non-Mass Breast Lesions on Ultrasound: Feature Exploration and Multimode Ultrasonic Diagnosis. Ultrasound Med Biol 44(8):1703–1711
Qu XX, Song Y, Zhang YH, Qing HM (2019) Value of Ultrasonic Elastography and Conventional Ultrasonography in the Differential Diagnosis of Non-Mass-like Breast Lesions. Ultrasound Med Biol 45(6):1358–1366
Park KW, Park S, Shon I et al (2021) Non-mass lesions detected by breast US: stratification of cancer risk for clinical management. Eur Radiol 31(3):1693–1706
Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W (2003) Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med 26(3):172–181
Buri M, Tanadini LG, Hothorn T, Curt A (2022) Unbiased Recursive Partitioning Enables Robust and Reliable Outcome Prediction in Acute Spinal Cord Injury. J Neurotrauma 39(3–4):266–276
Ciurea A, Calin A, Ciortea C, Dudea SM (2015) Ultrasound in the diagnosis of papillary breast lesions. Med Ultrason 17(3):392–397
Leong PW, Chotai NC, Kulkarni S (2018) Imaging Features of Inflammatory Breast Disorders: A Pictorial Essay. Korean J Radiol 19(1):5–14
Catanzariti F, Avendano D, Cicero G et al (2021) High-risk lesions of the breast: concurrent diagnostic tools and management recommendations. Insights Imaging 12:63. https://doi.org/10.1186/s13244-021-01005-6
Duric N, Sak M, Littrup PJ (2021) The Potential Role of the Fat-Glandular Interface (FGI) in Breast Carcinogenesis: Results from an Ultrasound Tomography (UST) Study. J Clin Med. https://doi.org/10.3390/jcm10235615
Littrup PJ, Duric N, Sak M et al (2021) The Fat-glandular Interface and Breast Tumor Locations: Appearances on Ultrasound Tomography Are Supported by Quantitative Peritumoral Analyses. J Breast Imaging 3:455–464
Kim WH, Li M, Han W, Ryu HS, Moon WK (2016) The Spatial Relationship of Malignant and Benign Breast Lesions with Respect to the Fat-Gland Interface on Magnetic Resonance Imaging. Sci RepDOI. https://doi.org/10.1038/srep39085
Zhu W, Harvey S, Macura KJ, Euhus DM, Artemov D (2017) Invasive Breast Cancer Preferably and Predominantly Occurs at the Interface Between Fibroglandular and Adipose Tissue. Clin Breast Cancer 17(1):e11–e18
Watanabe T, Yamaguchi T, Tsunoda H et al (2017) Ultrasound Image Classification of Ductal Carcinoma In Situ (DCIS) of the Breast: Analysis of 705 DCIS Lesions. Ultrasound Med Biol 43(5):918–925
Kim HR, Jung HK (2018) Histopathology findings of non-mass cancers on breast ultrasound. Acta Radiol OpenDOI. https://doi.org/10.1177/2058460118774957
Teboul M (2010) Advantages of Ductal Echography (DE) over conventional breast investigation in the diagnosis of breast malignancies. Med Ultrason 12(1):32–42
Tot T (2005) DCIS, cytokeratins, and the theory of the sick lobe. Virchows Arch 447(1):1–8
Choi JS, Han BK, Ko EY, Ko ES, Shin JH, Kim GR (2016) Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol 26(10):3542–3549
Barr RG, Engel A, Kim S, et al (2023) Improved Breast 2D SWE Algorithm to Eliminate False-Negative Cases. Invest Radiol Publish Ahead of Print: https://doi.org/10.1097/rli.0000000000000972
Funding
This study has received funding by the Natural Science Foundation Project of Shanghai Science and Technology Commission (No. 22ZR1400100).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Hansheng Xia.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
retrospective
-
diagnostic or prognostic study
-
performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Jing, L., Yan, L. et al. A conditional inference tree model for predicting cancer risk of non-mass lesions detected on breast ultrasound. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10504-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10504-7