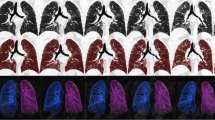
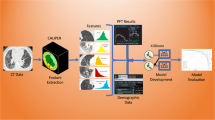
Abstract
Objectives
To evaluate the relationship of changes in the deep learning–based CT quantification of interstitial lung disease (ILD) with changes in forced vital capacity (FVC) and visual assessments of ILD progression, and to investigate their prognostic implications.
Methods
This study included ILD patients with CT scans at intervals of over 2 years between January 2015 and June 2021. Deep learning–based texture analysis software was used to segment ILD findings on CT images (fibrosis: reticular opacity + honeycombing cysts; total ILD extent: ground-glass opacity + fibrosis). Patients were grouped according to the absolute decline of predicted FVC (< 5%, 5–10%, and ≥ 10%) and ILD progression assessed by thoracic radiologists, and their quantification results were compared among these groups. The associations between quantification results and survival were evaluated using multivariable Cox regression analysis.
Results
In total, 468 patients (239 men; 64 ± 9.5 years) were included. Fibrosis and total ILD extents more increased in patients with larger FVC decline (p < .001 in both). Patients with ILD progression had higher fibrosis and total ILD extent increases than those without ILD progression (p < .001 in both). Increases in fibrosis and total ILD extent were significant prognostic factors when adjusted for absolute FVC declines of ≥ 5% (hazard ratio [HR] 1.844, p = .01 for fibrosis; HR 2.484, p < .001 for total ILD extent) and ≥ 10% (HR 2.918, p < .001 for fibrosis; HR 3.125, p < .001 for total ILD extent).
Conclusion
Changes in ILD CT quantification correlated with changes in FVC and visual assessment of ILD progression, and they were independent prognostic factors in ILD patients.
Clinical relevance statement
Quantifying the CT features of interstitial lung disease using deep learning techniques could play a key role in defining and predicting the prognosis of progressive fibrosing interstitial lung disease.
Key Points
• Radiologic findings on high-resolution CT are important in diagnosing progressive fibrosing interstitial lung disease.
• Deep learning–based quantification results for fibrosis and total interstitial lung disease extents correlated with the decline in forced vital capacity and visual assessments of interstitial lung disease progression, and emerged as independent prognostic factors.
• Deep learning–based interstitial lung disease CT quantification can play a key role in diagnosing and prognosticating progressive fibrosing interstitial lung disease.



Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- CTD-ILD:
-
Connective tissue disease–associated interstitial lung disease
- DLco:
-
Diffusing capacity of the lung for carbon monoxide
- FVC:
-
Forced vital capacity
- GGO:
-
Ground-glass opacity
- HR:
-
Hazard ratio
- HRCT:
-
High-resolution CT
- ILD:
-
Interstitial lung disease
- IQR:
-
Interquartile range
- IPF:
-
Idiopathic pulmonary fibrosis
- NSIP:
-
Nonspecific interstitial pneumonia
- PF-ILD:
-
Progressive fibrosing interstitial lung disease
- PFT:
-
Pulmonary function test
- UIP:
-
Usual interstitial pneumonia
References
Raghu G, Remy-Jardin M, Myers JL et al (2018) Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 198:e44–e68
Lynch DA, Sverzellati N, Travis WD et al (2018) Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 6:138–153
George PM, Patterson CM, Reed AK, Thillai M (2019) Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir Med 7:271–282
Kim HJ, Perlman D, Tomic R (2015) Natural history of idiopathic pulmonary fibrosis. Respir Med 109:661–670
George PM, Spagnolo P, Kreuter M et al (2020) Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 8:925–934
Wijsenbeek M, Kreuter M, Olson A et al (2019) Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin 35:2015–2024
Flaherty KR, Wells AU, Cottin V et al (2019) Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 381:1718–1727
Goldin JG (2018) Predicting outcome in idiopathic pulmonary fibrosis using automated computed tomography analysis. Am J Respir Crit Care Med 198:701–702
Richeldi L, Ryerson CJ, Lee JS et al (2012) Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax 67:407–411
du Bois RM, Weycker D, Albera C et al (2011) Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 184:1382–1389
Zappala CJ, Latsi PI, Nicholson AG et al (2010) Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 35:830–836
Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK (2003) Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 168:538–542
Jegal Y, Kim DS, Shim TS et al (2005) Physiology is a stronger predictor of survival than pathology in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 171:639–644
Latsi PI, du Bois RM, Nicholson AG et al (2003) Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med 168:531–537
Flaherty KR, Mumford JA, Murray S et al (2003) Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 168:543–548
Salisbury ML, Gu T, Murray S et al (2019) Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory. Chest 155:699–711
Walsh SL, Sverzellati N, Devaraj A, Wells AU, Hansell DM (2012) Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur Radiol 22:1672–1679
King TE, Bradford WZ, Castro-Bernardini S et al (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370:2083–2092
Maher TM, Corte TJ, Fischer A et al (2020) Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 8:147–157
Richeldi L, du Bois RM, Raghu G et al (2014) Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370:2071–2082
Behr J, Neuser P, Prasse A et al (2017) Exploring efficacy and safety of oral Pirfenidone for progressive, non-IPF lung fibrosis (RELIEF) - a randomized, double-blind, placebo-controlled, parallel group, multi-center, phase II trial. BMC Pulm Med 17:122
Ehteshami Bejnordi B, Veta M, Johannes van Diest P et al (2017) Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318:2199–2210
Esteva A, Kuprel B, Novoa RA et al (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542:115–118
Gulshan V, Peng L, Coram M et al (2016) Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316:2402–2410
Ting DSW, Cheung CY-L, Lim G et al (2017) Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 318:2211–2223
Lee SM, Seo JB, Yun J et al (2019) Deep learning applications in chest radiography and computed tomography: current state of the art. J Thorac Imaging 34:75
González G, Ash SY, Vegas-Sánchez-Ferrero G et al (2018) Disease staging and prognosis in smokers using deep learning in chest computed tomography. Am J Respir Crit Care Med 197:193–203
Lakhani P, Sundaram B (2017) Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology 284:574–582
Cicero M, Bilbily A, Colak E et al (2017) Training and validating a deep convolutional neural network for computer-aided detection and classification of abnormalities on frontal chest radiographs. Invest Radiol 52:281–287
Hwang EJ, Park S, Jin K-N et al (2019) Development and validation of a deep learning–based automated detection algorithm for major thoracic diseases on chest radiographs. JAMA Netw Open 2:e191095
Park HJ, Lee SM, Song JW et al (2016) Texture-based automated quantitative assessment of regional patterns on initial CT in patients with idiopathic pulmonary fibrosis: relationship to decline in forced vital capacity. AJR Am J Roentgenol 207:976–983
Kim HJ, Brown MS, Chong D et al (2015) Comparison of the quantitative CT imaging biomarkers of idiopathic pulmonary fibrosis at baseline and early change with an interval of 7 months. Acad Radiol 22:70–80
Goldin JG, Kim GHJ, Tseng C-H et al (2018) Longitudinal changes in quantitative interstitial lung disease on computed tomography after immunosuppression in the Scleroderma Lung Study II. Ann Am Thorac Soc 15:1286–1295
Park B, Park H, Lee SM, Seo JB, Kim N (2019) Lung segmentation on HRCT and volumetric CT for diffuse interstitial lung disease using deep convolutional neural networks. J Digit Imaging 32(6):1019–1026
Kim MS, Choe J, Hwang HJ et al (2022) Interstitial lung abnormalities (ILA) on routine chest CT: comparison of radiologists’ visual evaluation and automated quantification. Eur J Radiol 157:110564
Graham BL, Steenbruggen I, Miller MR et al (2019) Standardization of Spirometry 2019 Update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 200:e70–e88
Raghu G, Remy-Jardin M, Richeldi L et al (2022) Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 205:e18–e47
Lynch DA, Godwin JD, Safrin S et al (2005) High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med 172:488–493
Sumikawa H, Johkoh T, Colby TV et al (2008) Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med 177:433–439
Acknowledgements
The authors would like to acknowledge Andrew Dombrowski, Ph.D. (Compecs, Inc.) for his assistance in English editing.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jin Mo Goo.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
Activities not related to the present article—Jin Mo Goo reports research grants from LG Electronics and CoreLine Soft; Jong Hyuk Lee reports research grants from CoreLine Soft and consulting fee from Radisen.
Other authors (Seok Young Koh, Hyungin Park) have no conflicts of interest.
Statistics and biometry
One of the authors has significant statistical expertise (Jong Hyuk Lee).
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
The Institutional Review Board of Seoul National University Hospital approval was obtained (IRB No. H-2112-040-1279).
Study subjects or cohorts overlap
The study participants have not been reported before.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Seok Young Koh and Jong Hyuk Lee contributed equally to this research as first authors.
Supplementary information
ESM 1
(PDF 310 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koh, S.Y., Lee, J.H., Park, H. et al. Value of CT quantification in progressive fibrosing interstitial lung disease: a deep learning approach. Eur Radiol (2023). https://doi.org/10.1007/s00330-023-10483-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-023-10483-9