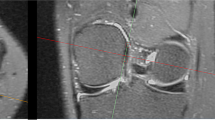
Abstract
Objective
To investigate the potential applicability of AI-assisted compressed sensing (ACS) in knee MRI to enhance and optimize the scanning process.
Methods
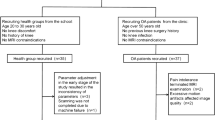
Volunteers and patients with sports-related injuries underwent prospective MRI scans with a range of acceleration techniques. The volunteers were subjected to varied ACS acceleration levels to ascertain the most effective level. Patients underwent scans at the determined optimal 3D-ACS acceleration level, and 3D compressed sensing (CS) and 2D parallel acquisition technology (PAT) scans were performed. The resultant 3D-ACS images underwent 3.5 mm/2.0 mm multiplanar reconstruction (MPR). Experienced radiologists evaluated and compared the quality of images obtained by 3D-ACS-MRI and 3D-CS-MRI, 3.5 mm/2.0 mm MPR and 2D-PAT-MRI, diagnosed diseases, and compared the results with the arthroscopic findings. The diagnostic agreement was evaluated using Cohen’s kappa correlation coefficient, and both absolute and relative evaluation methods were utilized for objective assessment.
Results
The study involved 15 volunteers and 53 patients. An acceleration factor of 10.69 × was identified as optimal. The quality evaluation showed that 3D-ACS provided poorer bone structure visualization, and improved cartilage visualization and less satisfactory axial images with 3.5 mm/2.0 mm MPR than 2D-PAT. In terms of objective evaluation, the relative evaluation yielded satisfactory results across different groups, while the absolute evaluation revealed significant variances in most features. Nevertheless, high levels of diagnostic agreement (κ: 0.81–0.94) and accuracy (0.83–0.98) were observed across all diagnoses.
Conclusion
ACS technology presents significant potential as a replacement for traditional CS in 3D-MRI knee scans, allowing thinner MPRs and markedly faster scans without sacrificing diagnostic accuracy.
Clinical relevance statement
3D-ACS-MRI of the knee can be completed in the 160 s with good diagnostic consistency and image quality. 3D-MRI-MPR can replace 2D-MRI and reconstruct images with thinner slices, which helps to optimize the current MRI examination process and shorten scanning time.
Key Points
• AI-assisted compressed sensing technology can reduce knee MRI scan time by over 50%.
• 3D AI-assisted compressed sensing MRI and related multiplanar reconstruction can replace traditional accelerated MRI and yield thinner 2D multiplanar reconstructions.
• Successful application of 3D AI-assisted compressed sensing MRI can help optimize the current knee MRI process.






Similar content being viewed by others
Abbreviations
- ACS:
-
AI-assisted compressed sensing
- CS:
-
Compressed sensing
- HF:
-
Half Fourier
- MPR:
-
Multiplanar reconstruction
- MSK:
-
Musculoskeletal
- PAT:
-
Parallel acquisition technology
- PD-FSE-FS:
-
Proton density-weighted fast spin‒echo with fat saturation
- PSNR:
-
Peak signal-to-noise ratio
- SSIM:
-
Structural similarity index
References
Morrison WB, Major N (2015) The knee. Top Magn Reson Imaging 24(4):193–203. https://doi.org/10.1097/rmr.0000000000000059
Naraghi AM, White LM (2016) Imaging of athletic injuries of knee ligaments and menisci: sports imaging series. Radiology 281(1):23–40. https://doi.org/10.1148/radiol.2016152320
Mohankumar R, White LM, Naraghi A (2014) Pitfalls and pearls in MRI of the knee. AJR Am J Roentgenol 203(3):516–530. https://doi.org/10.2214/ajr.14.12969
Shahabpour M, Handelberg F, Casteleyn PP, Machiels F, Osteaux M (1997) Imaging in sports-medicine–knee. Eur J Radiol 26(1):23–45. https://doi.org/10.1016/s0720-048x(97)00054-5
Altahawi F, Pierce J, Aslan M, Li X, Winalski CS, Subhas N (2021) 3D MRI of the Knee. Semin Musculoskelet Radiol 25(3):455–467. https://doi.org/10.1055/s-0041-1730400
Shakoor D, Guermazi A, Kijowski R et al (2019) Cruciate ligament injuries of the knee: a meta-analysis of the diagnostic performance of 3D MRI. J Magn Reson Imaging 50(5):1545–1560. https://doi.org/10.1002/jmri.26713
Wei H, Lin H, Qin L et al (2019) Quantitative susceptibility mapping of articular cartilage in patients with osteoarthritis at 3T. J Magn Reson Imaging: JMRI 49(6):1665–1675. https://doi.org/10.1002/jmri.26535
Montalt-Tordera J, Muthurangu V, Hauptmann A, Steeden JA (2021) Machine learning in magnetic resonance imaging: image reconstruction. Phys Med 83:79–87. https://doi.org/10.1016/j.ejmp.2021.02.020
Gao T, Lu Z, Wang F, Zhao H, Wang J, Pan S (2021) Using the compressed sensing technique for lumbar vertebrae imaging: comparison with conventional parallel imaging. Curr Med Imaging 17(8):1010–1017. https://doi.org/10.2174/1573405617666210126155814
Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H (2018) Artificial intelligence in radiology. Nat Rev Cancer 18(8):500–510. https://doi.org/10.1038/s41568-018-0016-5
Chen X, Wang X, Zhang K et al (2022) Recent advances and clinical applications of deep learning in medical image analysis. Med Image Anal 79:102444. https://doi.org/10.1016/j.media.2022.102444
Sheng R-F, Zheng L-Y, Jin K-P et al (2021) Single-breath-hold T2WI liver MRI with deep learning-based reconstruction: a clinical feasibility study in comparison to conventional multi-breath-hold T2WI liver MRI. Magn Reson Imaging 81:75–81. https://doi.org/10.1016/j.mri.2021.06.014
Sui H, Gong Y, Liu L et al (2023) Comparison of artificial intelligence-assisted compressed sensing (ACS) and routine two-dimensional sequences on lumbar spine imaging. J Pain Res 16:257–67. https://doi.org/10.2147/jpr.S388219
Sun Z, Wu P, Cui Y, et al. (2023) Deep-learning models for detection and localization of visible clinically significant prostate cancer on multi-parametric MRI. J Magn Reson Imaging https://doi.org/10.1002/jmri.28608 epub ahead of print
Zhao Y, Peng C, Wang S, Liang X, Meng X (2022) The feasibility investigation of AI-assisted compressed sensing in kidney MR imaging: an ultra-fast T2WI imaging technology. BMC Med Imaging 22(1):1–6. https://doi.org/10.1186/s12880-022-00842-1
Zhao Y, Peng C, Wang S, Liang X, Meng X (2022) The feasibility investigation of AI -assisted compressed sensing in kidney MR imaging: an ultra-fast T2WI imaging technology. BMC Med Imaging 22(1):119. https://doi.org/10.1186/s12880-022-00842-1
Wang Z, Bovik AC, Sheikh HR, Simoncelli EP (2004) Image quality assessment: from error visibility to structural similarity. IEEE Trans Image Process: Publ IEEE Signal Process Soc 13(4):600–612. https://doi.org/10.1109/tip.2003.819861
Zhou W, Bovik AC (2002) A universal image quality index. IEEE Signal Process Lett 9(3):81–84. https://doi.org/10.1109/97.995823
Xue W, Zhang L, Mou X, Bovik AC (2014) Gradient magnitude similarity deviation: a highly efficient perceptual image quality index. IEEE Trans Image Process 23(2):684–695. https://doi.org/10.1109/TIP.2013.2293423
Zhang L, Zhang L, Mou X, Zhang D (2011) FSIM: a feature similarity index for image quality assessment. IEEE Trans Image Process 20(8):2378–2386. https://doi.org/10.1109/TIP.2011.2109730
Andre JB, Bresnahan BW, Mossa-Basha M et al (2015) Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. J Am College Radiol: JACR 12(7):689–695. https://doi.org/10.1016/j.jacr.2015.03.007
Kim M, Lee SM, Park C et al (2022) Deep learning-enhanced parallel imaging and simultaneous multislice acceleration reconstruction in knee MRI. Invest Radiol 57(12):826–833. https://doi.org/10.1097/rli.0000000000000900
Foreman SC, Neumann J, Han J et al (2022) Deep learning-based acceleration of compressed sense MR imaging of the ankle. Eur Radiol 32(12):8376–8385. https://doi.org/10.1007/s00330-022-08919-9
Keller G, Estler A, Herrmann J et al (2023) Prospective intraindividual comparison of a standard 2D TSE MRI protocol for ankle imaging and a deep learning-based 2D TSE MRI protocol with a scan time reduction of 48. Radiol Med 128(3):347–356. https://doi.org/10.1007/s11547-023-01604-x
Han Y, Sunwoo L, Ye JC (2020) k -space deep learning for accelerated MRI. IEEE Trans Med Imaging 39(2):377–386. https://doi.org/10.1109/tmi.2019.2927101
Hong GQ, Wei YT, Morley WAW et al (2023) Dual-domain accelerated MRI reconstruction using transformers with learning-based undersampling. Comput Med Imaging Graph 106:102206. https://doi.org/10.1016/j.compmedimag.2023.102206
Lin DJ, Walter SS, Fritz J (2023) Artificial intelligence-driven ultra-fast superresolution MRI: 10-fold accelerated musculoskeletal turbo spin echo MRI within reach. Invest Radiol 58(1):28–42. https://doi.org/10.1097/rli.0000000000000928
Johnson PM, Lin DJ, Zbontar J et al (2023) Deep learning reconstruction enables prospectively accelerated clinical knee MRI. Radiology 307(2):e220425. https://doi.org/10.1148/radiol.220425
Oei EHG, van Zadelhoff TA, Eijgenraam SM, Klein S, Hirvasniemi J, van der Heijden RA (2021) 3D MRI in osteoarthritis. Semin Musculoskelet Radiol 25(3):468–479. https://doi.org/10.1055/s-0041-1730911
Jones BC, Ahlawat S, Fayad LM (2021) 3D MRI in musculoskeletal oncology. Semin Musculoskelet Radiol 25(3):418–424. https://doi.org/10.1055/s-0041-1730399
Ezzati F, Chalian M, Pezeshk P (2021) 3D MRI of the rheumatic diseases. Semin Musculoskelet Radiol 25(3):425–432. https://doi.org/10.1055/s-0041-1731058
Funding
This study received financial support from the National Natural Science Foundation of China (82171927), the Beijing Natural Science Foundation (7212126), and the Beijing New Health Industry Development Foundation (XM2020-02–006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Huishu Yuan.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: United Imaging. Among the authors, Yunxin Yang and Peng Wu are employees of the company, and are mainly involved in the experimental protocol design, MRI scanning sequence debugging, and manuscript revision of this study. To ensure the authenticity and fairness of the results, we do not allow company personnel to touch the data processing process. The investigators of this study did not receive any compensation.
The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Xiaoyi Wen, from the Institute of Statistics and Big Data, Renmin University of China, Beijing, People’s Republic of China, was responsible for all statistical content of this study and is listed as one of the authors.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained. The medical science research ethics committee of Peking University Third Hospital approved this prospective study.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
• prospective
-
• diagnostic or prognostic study
-
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ni, M., He, M., Yang, Y. et al. Application research of AI-assisted compressed sensing technology in MRI scanning of the knee joint: 3D-MRI perspective. Eur Radiol 34, 3046–3058 (2024). https://doi.org/10.1007/s00330-023-10368-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10368-x