Abstract
Objectives
This study aimed to propose a deep learning (DL)–based framework for identifying the composition of thyroid nodules and assessing their malignancy risk.
Methods
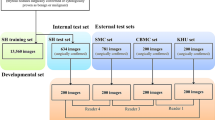
We conducted a retrospective multicenter study using ultrasound images from four hospitals. Convolutional neural network (CNN) models were constructed to classify ultrasound images of thyroid nodules into solid and non-solid, as well as benign and malignant. A total of 11,201 images of 6784 nodules were used for training, validation, and testing. The area under the receiver-operating characteristic curve (AUC) was employed as the primary evaluation index.
Results
The models had AUCs higher than 0.91 in the benign and malignant grading of solid thyroid nodules, with the Inception-ResNet AUC being the highest at 0.94. In the test set, the best algorithm for identifying benign and malignant thyroid nodules had a sensitivity of 0.88, and a specificity of 0.86. In the human vs. DL test set, the best algorithm had a sensitivity of 0.93, and a specificity of 0.86. The Inception-ResNet model performed better than the senior physicians (p < 0.001). The sensitivity and specificity of the optimal model based on the external test set were 0.90 and 0.75, respectively.
Conclusions
This research demonstrates that CNNs can assist thyroid nodule diagnosis and reduce the rate of unnecessary fine-needle aspiration (FNA).
Clinical relevance statement
High-resolution ultrasound has led to increased detection of thyroid nodules. This results in unnecessary fine-needle aspiration and anxiety for patients whose nodules are benign. Deep learning can solve these problems to some extent.
Key Points
• Thyroid solid nodules have a high probability of malignancy.
• Our models can improve the differentiation between benign and malignant solid thyroid nodules.
• The differential performance of one model was superior to that of senior radiologists. Applying this could reduce the rate of unnecessary fine-needle aspiration of solid thyroid nodules.





Similar content being viewed by others
Abbreviations
- ACR:
-
American College of Radiology
- AI:
-
Artificial intelligence
- AUC:
-
Area under the receiver-operating characteristic curve
- CEUS:
-
Contrast-enhanced ultrasound
- CI:
-
Confidence intervals
- CNN:
-
Convolutional neural network
- DL:
-
Deep learning
- FNA:
-
Fine-needle aspiration
- Grad-CAM:
-
Gradient class activation mapping
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- TI-RADS:
-
Thyroid Image Radiology and Data System
- US:
-
Ultrasonography
- US-FNAB:
-
Ultrasound-guided fine-needle aspiration biopsy
References
Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L (2016) Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 375:614–617
Feng R-M, Zong Y-N, Cao S-M, Xu R-H (2019) Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond) 39:22
Ma X, Zhang L (2022) Diagnosis of thyroid nodules based on image enhancement and deep neural networks. Comput Intell Neurosci 2022:5582029
Zhang X, Lee VCS, Rong J, Liu F, Kong H (2022) Multi-channel convolutional neural network architectures for thyroid cancer detection. PLoS One 17:e0262128
Singh Ospina N, Brito JP, Maraka S et al (2016) Diagnostic accuracy of ultrasound-guided fine needle aspiration biopsy for thyroid malignancy: systematic review and meta-analysis. Endocrine 53:651–661
Yoon JH, Moon HJ, Kim E-K, Kwak JY (2011) Inadequate cytology in thyroid nodules: should we repeat aspiration or follow-up? Ann Surg Oncol 18:1282–1289
Chan WK, Sun JH, Liou MJ et al (2021) Using deep convolutional neural networks for enhanced ultrasonographic image diagnosis of differentiated thyroid cancer. Biomedicines 9:1771
Zhang D, Jiang F, Yin R et al (2021) A review of the role of the S-detect computer-aided diagnostic ultrasound system in the evaluation of benign and malignant breast and thyroid masses. Med Sci Monit 27:e931957
Zhao D, Jing Y, Lin X, Zhang B (2021) The value of color Doppler ultrasound in the diagnosis of thyroid nodules: a systematic review and meta-analysis. Gland Surg 10:3369–3377
Zhou Y, Chen H, Qiang J, Wang D (2021) Systematic review and meta-analysis of ultrasonic elastography in the diagnosis of benign and malignant thyroid nodules. Gland Surg 10:2734–2744
Li W, Sun Y, Xu H, Shang W, Dong A (2022) Systematic review and meta-analysis of American College of Radiology TI-RADS inter-reader reliability for risk stratification of thyroid nodules. Front Oncol 12:840516
Nguyen DT, Kang JK, Pham TD, Batchuluun G, Park KR (2020) Ultrasound image-based diagnosis of malignant thyroid nodule using artificial intelligence. Sensors (Basel) 20:1822
Modi L, Sun W, Shafizadeh N et al (2020) Does a higher American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) score forecast an increased risk of malignancy? A correlation study of ACR TI-RADS with FNA cytology in the evaluation of thyroid nodules. Cancer Cytopathol 128:470–481
Wang J, Jiang J, Zhang D et al (2022) An integrated AI model to improve diagnostic accuracy of ultrasound and output known risk features in suspicious thyroid nodules. Eur Radiol 32:2120–2129
Hoang JK, Middleton WD, Farjat AE et al (2018) Reduction in thyroid nodule biopsies and improved accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 287:185–193
Sharifi Y, Shafiei S, Tabesh H et al (2022) Observation variation in ultrasonography assessment of thyroid nodules. Asia Ocean J Nucl Med Biol 10:28–35
Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L (2017) European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J 6:225–237
Kwak JY, Han KH, Yoon JH et al (2011) Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 260:892–899
Li W, Zhu Q, Jiang Y et al (2017) Partially cystic thyroid nodules in ultrasound-guided fine needle aspiration: prevalence of thyroid carcinoma and ultrasound features. Medicine (Baltimore) 96:e8689
Fang F, Gong Y, Liao L et al (2021) Value of contrast-enhanced ultrasound in partially cystic papillary thyroid carcinomas. Front Endocrinol (Lausanne) 12:783670
Brito JP, Gionfriddo MR, Al Nofal A et al (2014) The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab 99:1253–1263
Remonti LR, Kramer CK, Leitão CB, Pinto LC, Gross JL (2015) Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 25:538–550
Hoang JK, Middleton WD, Tessler FN (2021) Update on ACR TI-RADS: successes, challenges, and future directions, from the AJR Special Series on Radiology Reporting and Data Systems. AJR Am J Roentgenol 216:570–578
Peng Y, Zhou W, Zhan WW, Xu SY (2017) Ultrasonographic assessment of differential diagnosis between degenerating cystic thyroid nodules and papillary thyroid microcarcinomas. World J Surg 41:2538–2544
Peng Q, Niu C, Zhang Q, Zhang M, Chen S, Peng Q (2019) Mummified thyroid nodules: conventional and contrast-enhanced ultrasound features. J Ultrasound Med 38:441–452
Chen S, Tang K, Gong Y et al (2022) Value of contrast-enhanced ultrasound in mummified thyroid nodules. Front Endocrinol 13:850698
Ruan J, Xu X, Cai Y et al (2022) A practical CEUS thyroid reporting system for thyroid nodules. Radiology 305:149–159
Wu GG, Lv WZ, Yin R et al (2021) Deep learning based on ACR TI-RADS can improve the differential diagnosis of thyroid nodules. Front Oncol 11:575166
Zhu YC, Jin PF, Bao J, Jiang Q, Wang X (2021) Thyroid ultrasound image classification using a convolutional neural network. Ann Transl Med 9:1526
Chen Y, Gao Z, He Y et al (2022) An artificial intelligence model based on ACR TI-RADS characteristics for US diagnosis of thyroid nodules. Radiology 303:613–619
Yao J-C, Wang T, Hou G-H et al (2021) AI detection of mild COVID-19 pneumonia from chest CT scans. Eur Radiol 31:7192–7201
Wang Y, Guan Q, Lao I et al (2019) Using deep convolutional neural networks for multi-classification of thyroid tumor by histopathology: a large-scale pilot study. Ann Transl Med 7:468
Yu J, Deng Y, Liu T et al (2020) Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nat Commun 11:4807
Zhao CK, Ren TT, Yin YF et al (2021) A comparative analysis of two machine learning-based diagnostic patterns with thyroid imaging reporting and data system for thyroid nodules: diagnostic performance and unnecessary biopsy rate. Thyroid 31:470–481
Tessler FN, Middleton WD, Grant EG et al (2017) ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol 14:587–595
Yunus M, Ahmed Z (2010) Significance of ultrasound features in predicting malignant solid thyroid nodules: need for fine-needle aspiration. J Pak Med Assoc 60:848–853
Ren S, He K, Girshick R, Sun J (2017) Faster R-CNN: towards real-time object detection with region proposal networks. IEEE Trans Pattern Anal Mach Intell 39:1137–1149
Russ G (2016) Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography 35:25–38
Kim J, Gosnell JE, Roman SA (2020) Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol 16:17–29
Pitoia F, Miyauchi A (2016) 2015 American Thyroid Association guidelines for thyroid nodules and differentiated thyroid cancer and their implementation in various care settings. Thyroid 26:319–321
Dighe M, Barr R, Bojunga J et al (2017) Thyroid ultrasound: state of the art part 1 - thyroid ultrasound reporting and diffuse thyroid diseases. Med Ultrason 19:79–93
Dietrich CF, Müller T, Bojunga J et al (2018) Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol 44:14–36
Trimboli P, Dietrich CF, David E et al (2018) Ultrasound and ultrasound-related techniques in endocrine diseases. Minerva Endocrinol 43:333–340
Hoang JK, Middleton WD, Langer JE et al (2021) Comparison of thyroid risk categorization systems and fine-needle aspiration recommendations in a multi-institutional thyroid ultrasound registry. J Am Coll Radiol 18:1605–1613
Qadan L, Ahmed A, Kapila K (2019) Thyroid ultrasound reports: deficiencies and recommendations. Med Princ Pract 28:280–283
Buda M, Wildman-Tobriner B, Hoang JK et al (2019) Management of thyroid nodules seen on US images: deep learning may match performance of radiologists. Radiology 292:695–701
Tuttle RM, Fagin JA, Minkowitz G et al (2017) Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 143:1015–1020
Xia J, Chen H, Li Q et al (2017) Ultrasound-based differentiation of malignant and benign thyroid nodules: an extreme learning machine approach. Comput Methods Programs Biomed 147:37–49
Ma J, Wu F, Zhu J, Xu D, Kong D (2017) A pre-trained convolutional neural network based method for thyroid nodule diagnosis. Ultrasonics 73:221–230
Tessler FN, Middleton WD, Grant EG (2018) Thyroid Imaging Reporting and Data System (TI-RADS): a user’s guide. Radiology 287:1082
Gao L, Liu R, Jiang Y et al (2018) Computer-aided system for diagnosing thyroid nodules on ultrasound: a comparison with radiologist-based clinical assessments. Head Neck 40:778–783
Li X, Zhang S, Zhang Q et al (2019) Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: a retrospective, multicohort, diagnostic study. Lancet Oncol 20:193–201
Swan KZ, Thomas J, Nielsen VE, Jespersen ML, Bonnema S (2022) External validation of AIBx, an artificial intelligence model for risk stratification, in thyroid nodules. Eur Thyroid J 11:e210129
Acknowledgements
The authors thank ultrasound doctors at the Department of Ultrasound, Zhejiang Cancer Hospital, Shenzhen People’s Hospital, Shanghai Tenth People’s Hospital, and Taizhou Cancer Hospital for data collection. Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82071946), “Pioneer” and “Leading Goose” R&D Program of Zhejiang (No. 2023C04039), Research Program of National Health Commision Capacity Building and Continuing Education Center (CSJRZC2021JJSJ001), the Natural Science Foundation of Zhejiang Province (No. LZY21F030001), and the Research Program of Zhejiang Provincial Department of Health (No. 2021KY099, 2022KY110, and 2023KY066).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Dong Xu.
Conflict of Interest
One of the authors (Yitao Jiang) is an employee of Illuminate, LLC, Guangdong, China. The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and Biometry
No complex statistical methods were necessary for this paper.
Informed Consent
Written informed consent was waived by the institutional review board.
Ethical Approval
This study was approved by the Ethics Committee of the Cancer Hospital of The University of Chinese Academy of Sciences, Shenzhen People’s Hospital, Shanghai Tenth People’s Hospital, and Taizhou Cancer Hospital.
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
• Retrospective
• Diagnostic study
• Multicenter study
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C., Jiang, Y., Yao, J. et al. Deep learning to assist composition classification and thyroid solid nodule diagnosis: a multicenter diagnostic study. Eur Radiol 34, 2323–2333 (2024). https://doi.org/10.1007/s00330-023-10269-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10269-z