Abstract
Objectives
To develop a radiomics model in contrast-enhanced cone-beam breast CT (CE-CBBCT) for preoperative prediction of axillary lymph node (ALN) status and metastatic burden of breast cancer.
Methods
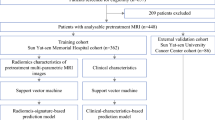
Two hundred and seventy-four patients who underwent CE-CBBCT examination with two scanners between 2012 and 2021 from two institutions were enrolled. The primary tumor was annotated in each patient image, from which 1781 radiomics features were extracted with PyRadiomics. After feature selection, support vector machine models were developed to predict ALN status and metastatic burden. To avoid overfitting on a specific patient subset, 100 randomly stratified splits were made to assign the patients to either training/fine-tuning or test set. Area under the receiver operating characteristic curve (AUC) of these radiomics models was compared to those obtained when training the models only with clinical features and combined clinical-radiomics descriptors. Ground truth was established by histopathology.
Results
One hundred and eighteen patients had ALN metastasis (N + (≥ 1)). Of these, 74 had low burden (N + (1~2)) and 44 high burden (N + (≥ 3)). The remaining 156 patients had none (N0). AUC values across the 100 test repeats in predicting ALN status (N0/N + (≥ 1)) were 0.75 ± 0.05 (0.67~0.93, radiomics model), 0.68 ± 0.07 (0.53~0.85, clinical model), and 0.74 ± 0.05 (0.67~0.88, combined model). For metastatic burden prediction (N + (1~2)/N + (≥ 3)), AUC values were 0.65 ± 0.10 (0.50~0.88, radiomics model), 0.55 ± 0.10 (0.40~0.80, clinical model), and 0.64 ± 0.09 (0.50~0.90, combined model), with all the ranges spanning 0.5. In both cases, the radiomics model was significantly better than the clinical model (both p < 0.01) and comparable with the combined model (p = 0.56 and 0.64).
Conclusions
Radiomics features of primary tumors could have potential in predicting ALN metastasis in CE-CBBCT imaging.
Clinical relevance statement
The findings support potential clinical use of radiomics for predicting axillary lymph node metastasis in breast cancer patients and addressing the limited axilla coverage of cone-beam breast CT.
Key Points
• Contrast-enhanced cone-beam breast CT-based radiomics could have potential to predict N0 vs. N + (≥ 1) and, to a limited extent, N + (1~2) vs. N + (≥ 3) from primary tumor, and this could help address the limited axilla coverage, pending future verifications on larger cohorts.
• The average AUC of radiomics and combined models was significantly higher than that of clinical models but showed no significant difference between themselves.
• Radiomics features descriptive of tumor texture were found informative on axillary lymph node status, highlighting a higher heterogeneity for tumor with positive axillary lymph node.




Similar content being viewed by others
Abbreviations
- ACOSOG:
-
American College of Surgeons Oncology Group
- ALN:
-
Axillary lymph node
- ALND:
-
Axillary lymph node dissection
- AUC:
-
Area under the curve
- CE-CBBCT:
-
Contrast-enhanced cone-beam breast CT
- FNB:
-
Fine-needle biopsy
- ICC:
-
Intraclass correlation coefficient
- IQR:
-
Interquartile range
- LoG:
-
Laplacian of Gaussian
- LASSO:
-
Least absolute shrinkage selection operator
- NME:
-
Non-mass enhancement
- ROC:
-
Receiver operating characteristic
- SLNB:
-
Sentinel lymph node biopsy
- SVM:
-
Support vector machine
- VOI:
-
Volume of interest
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33
Gradishar WJ, Moran MS, Abraham J et al (2022) Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 20:691–722
Giuliano AE, Ballman KV, McCall L et al (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 318:918–926
Zhu Y, Li X, Wang F et al (2018) Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging in characterization of axillary lymph nodes: preliminary animal experience. Magn Reson Imaging 52:46–52
Li H, Yin L, He N et al (2019) Comparison of comfort between cone beam breast computed tomography and digital mammography. Eur J Radiol 120:108674
Uhlig J, Uhlig A, Biggemann L, Fischer U, Lotz J, Wienbeck S (2019) Diagnostic accuracy of cone-beam breast computed tomography: a systematic review and diagnostic meta-analysis. Eur Radiol 29:1194–1202
Zhu Y, O'Connell AM, Ma Y et al (2022) Dedicated breast CT: state of the art-part I. historical evolution and technical aspects. Eur Radiol 32:1579–1589
Zhu Y, O'Connell AM, Ma Y et al (2022) Dedicated breast CT: state of the art-part II. clinical application and future outlook. Eur Radiol 32:2286–2300
Liu A, Ma Y, Yin L et al (2023) Comparison of malignant calcification identification between breast cone-beam computed tomography and digital mammography. Acta Radiol 64:962–970
Wienbeck S, Uhlig J, Luftner-Nagel S et al (2017) The role of cone-beam breast-CT for breast cancer detection relative to breast density. Eur Radiol 27:5185–5195
Wienbeck S, Fischer U, Luftner-Nagel S, Lotz J, Uhlig J (2018) Contrast-enhanced cone-beam breast-CT (CBBCT): clinical performance compared to mammography and MRI. Eur Radiol 28:3731–3741
Uhlig J, Fischer U, Biggemann L, Lotz J, Wienbeck S (2019) Pre- and post-contrast versus post-contrast cone-beam breast CT: can we reduce radiation exposure while maintaining diagnostic accuracy? Eur Radiol 29:3141–3148
Kang W, Zhong W, Su D (2021) The cone-beam breast computed tomography characteristics of breast non-mass enhancement lesions. Acta Radiol 62:1298–1308
Zhu Y, Zhang Y, Ma Y et al (2020) Cone-beam breast CT features associated with HER2/neu overexpression in patients with primary breast cancer. Eur Radiol 30:2731–2739
Ma Y, Liu A, O'Connell AM et al (2021) Contrast-enhanced cone beam breast CT features of breast cancers: correlation with immunohistochemical receptors and molecular subtypes. Eur Radiol 31:2580–2589
Wienbeck S, Uhlig J, Fischer U et al (2019) Breast lesion size assessment in mastectomy specimens: correlation of cone-beam breast-CT, digital breast tomosynthesis and full-field digital mammography with histopathology. Medicine (Baltimore) 98:e17082
Wang Y, Zhao M, Ma Y et al (2023) Accuracy of preoperative contrast-enhanced cone beam breast CT in assessment of residual tumor after neoadjuvant chemotherapy: a comparative study with breast MRI. Acad Radiol 30:1805–1815
Ma Y, Cao Y, Liu A et al (2019) A reliability comparison of cone-beam breast computed tomography and mammography: breast density assessment referring to the fifth edition of the BI-RADS atlas. Acad Radiol 26:752–759
Liu A, Yin L, Ma Y et al (2022) Quantitative breast density measurement based on three-dimensional images: a study on cone-beam breast computed tomography. Acta Radiol 63:1023–1031
O’Connell A, Conover DL, Zhang Y et al (2010) Cone-beam CT for breast imaging: Radiation dose, breast coverage, and image quality. AJR Am J Roentgenol 195:496–509
Scapicchio C, Gabelloni M, Barucci A, Cioni D, Saba L, Neri E (2021) A deep look into radiomics. Radiol Med 126:1296–1311
Yang J, Wang T, Yang L et al (2019) Preoperative prediction of axillary lymph node metastasis in breast cancer using mammography-based radiomics method. Sci Rep 9:4429
Yu FH, Wang JX, Ye XH, Deng J, Hang J, Yang B (2019) Ultrasound-based radiomics nomogram: a potential biomarker to predict axillary lymph node metastasis in early-stage invasive breast cancer. Eur J Radiol 119:108658
Dong Y, Feng Q, Yang W et al (2018) Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI. Eur Radiol 28:582–591
Caballo M, Hernandez AM, Lyu SH et al (2021) Computer-aided diagnosis of masses in breast computed tomography imaging: deep learning model with combined handcrafted and convolutional radiomic features. J Med Imaging (Bellingham) 8:024501
Caballo M, Pangallo DR, Sanderink W et al (2021) Multi-marker quantitative radiomics for mass characterization in dedicated breast CT imaging. Med Phys 48:313–328
Caballo M, Pangallo DR, Mann RM, Sechopoulos I (2020) Deep learning-based segmentation of breast masses in dedicated breast CT imaging: radiomic feature stability between radiologists and artificial intelligence. Comput Biol Med 118:103629
Ding J, Chen S, Serrano Sosa M et al (2022) Optimizing the peritumoral region size in radiomics analysis for sentinel lymph node status prediction in breast cancer. Acad Radiol 29:S223–S228
Wang D, Hu Y, Zhan C, Zhang Q, Wu Y, Ai T (2022) A nomogram based on radiomics signature and deep-learning signature for preoperative prediction of axillary lymph node metastasis in breast cancer. Front Oncol 12:940655
National Health Commission of the People’s Republic of China (2022) National guidelines for diagnosis and treatment of breast cancer 2022 in China (English version). Chin J Cancer Res 34:151–175
Ma Y, Liu A, Zhang Y et al (2022) Comparison of background parenchymal enhancement (BPE) on contrast-enhanced cone-beam breast CT (CE-CBBCT) and breast MRI. Eur Radiol 32:5773–5782
He N, Wu YP, Kong Y et al (2016) The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: a prospective study with 212 patients. Eur J Radiol 85:392–403
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107
Chitalia RD, Rowland J, McDonald ES et al (2020) Imaging phenotypes of breast cancer heterogeneity in preoperative breast dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) scans predict 10-year recurrence. Clin Cancer Res 26:862–869
Altman DG, Bland JM (2011) How to obtain the p value from a confidence interval. BMJ 343:d2304
Zhang J, Li L, Zhe X et al (2022) The diagnostic performance of machine learning-based radiomics of DCE-MRI in predicting axillary lymph node metastasis in breast cancer: a meta-analysis. Front Oncol 12:799209
Gong X, Guo Y, Zhu T, Peng X, Xing D, Zhang M (2022) Diagnostic performance of radiomics in predicting axillary lymph node metastasis in breast cancer: a systematic review and meta-analysis. Front Oncol 12:1046005
Eldaly AS, Avila FR, Torres-Guzman RA et al (2023) Radiomics and artificial intelligence in predicting axillary lymph node metastasis in breast cancer: a systematic review. Curr Med Imaging 19:564–578
Zhang X, Yang Z, Cui W et al (2021) Preoperative prediction of axillary sentinel lymph node burden with multiparametric MRI-based radiomics nomogram in early-stage breast cancer. Eur Radiol 31:5924–5939
Tan H, Wu Y, Bao F et al (2020) Mammography-based radiomics nomogram: a potential biomarker to predict axillary lymph node metastasis in breast cancer. Br J Radiol 93:20191019
Mao N, Yin P, Li Q et al (2020) Radiomics nomogram of contrast-enhanced spectral mammography for prediction of axillary lymph node metastasis in breast cancer: a multicenter study. Eur Radiol 30:6732–6739
Han L, Zhu Y, Liu Z et al (2019) Radiomic nomogram for prediction of axillary lymph node metastasis in breast cancer. Eur Radiol 29:3820–3829
Jiang M, Li CL, Luo XM et al (2022) Radiomics model based on shear-wave elastography in the assessment of axillary lymph node status in early-stage breast cancer. Eur Radiol 32:2313–2325
Gao Y, Luo Y, Zhao C et al (2021) Nomogram based on radiomics analysis of primary breast cancer ultrasound images: prediction of axillary lymph node tumor burden in patients. Eur Radiol 31:928–937
Liu C, Ding J, Spuhler K et al (2019) Preoperative prediction of sentinel lymph node metastasis in breast cancer by radiomic signatures from dynamic contrast-enhanced MRI. J Magn Reson Imaging 49:131–140
Chen H, Lan X, Yu T et al (2022) Development and validation of a radiogenomics model to predict axillary lymph node metastasis in breast cancer integrating MRI with transcriptome data: a multicohort study. Front Oncol 12:1076267
Papanikolaou N, Matos C, Koh DM (2020) How to develop a meaningful radiomic signature for clinical use in oncologic patients. Cancer Imaging 20:33
An C, Park YW, Ahn SS, Han K, Kim H, Lee SK (2021) Radiomics machine learning study with a small sample size: single random training-test set split may lead to unreliable results. PLoS One 16:e0256152
Akhtar M, Haider A, Rashid S, Al-Nabet ADMH (2019) Paget’s “seed and soil” theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol 26:69–74
McGranahan N, Swanton C (2017) Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168:613–628
Eifer M, Pinian H, Klang E et al (2022) FDG PET/CT radiomics as a tool to differentiate between reactive axillary lymphadenopathy following COVID-19 vaccination and metastatic breast cancer axillary lymphadenopathy: a pilot study. Eur Radiol 32:5921–5929
Caballo M, Mann R, Sechopoulos I (2018) Patient-based 4D digital breast phantom for perfusion contrast-enhanced breast CT imaging. Med Phys 45:4448–4460
Caballo M, Michielsen K, Fedon C, Sechopoulos I (2019) Towards 4D dedicated breast CT perfusion imaging of cancer: development and validation of computer simulated images. Phys Med Biol 64:245004
Fong W, Tan L, Tan C et al (2022) Predicting the risk of axillary lymph node metastasis in early breast cancer patients based on ultrasonographic-clinicopathologic features and the use of nomograms: a prospective single-center observational study. Eur Radiol 32:8200–8212
Pathmanathan N, Balleine RL (2013) Ki67 and proliferation in breast cancer. J Clin Pathol 66:512–516
Majidpoor J, Mortezaee K (2021) Angiogenesis as a hallmark of solid tumors-clinical perspectives. Cell Oncol (Dordr) 44:715–737
Bhat AA, Yousuf P, Wani NA et al (2021) Tumor microenvironment: an evil nexus promoting aggressive head and neck squamous cell carcinoma and avenue for targeted therapy. Signal Transduct Target Ther 6:12
Kalli S, Semine A, Cohen S, Naber SP, Makim SS, Bahl M (2018) American Joint Committee on Cancer’s Staging System for Breast Cancer, eighth edition: what the radiologist needs to know. Radiographics 38:1921–1933
Teichgraeber DC, Guirguis MS, Whitman GJ (2021) Breast cancer staging: updates in the AJCC cancer staging manual, 8th edition, and current challenges for radiologists, from the AJR special series on cancer staging. AJR Am J Roentgenol 217:278–290
Pesek S, Ashikaga T, Krag LE, Krag D (2012) The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J Surg 36:2239–2251
Singh A, Horng H, Chitalia R et al (2022) Resampling and harmonization for mitigation of heterogeneity in image parameters of baseline scans. Sci Rep 12:21505
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Sun Q, Lin X, Zhao Y et al (2020) Deep learning vs. radiomics for predicting axillary lymph node metastasis of breast cancer using ultrasound images: don’t forget the peritumoral region. Front. Oncol 10:53
Zheng X, Yao Z, Huang Y et al (2020) Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat Commun 11:1236
Funding
This study was supported by National Key R&D Program of China (No. 2021YFC2500400, 2021YFC2500402, 2017YFC0112600, 2017YFC0112601, 2017YFC0112605), National Natural Science Foundation of China (No. 81571671), Tianjin Science and Technology Major Project (No. 19ZXDBSY00080), Key Project of Tianjin Medical Industry (No. 16KG130), Tianjin Medical University Cancer Institute and Hospital Fund (B2118, B2219), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A). This study was also supported in part by the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (No. R01CA181171). The content is solely the responsibility of the authors and does not represent the official views of the NCI or the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhaoxiang Ye.
Conflict of Interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and Biometry
One of the authors (Marco Caballo) has significant statistical expertise.
Informed Consent
Data for this study was collected from two prospective clinical trials, named “Koning breast CT for breast imaging in China” and “The technical operations and standard clinical application protocol of cone-beam breast CT in diagnostic process of breast cancer”, in which written informed consent including permission to re-use the data for any further retrospective analysis was obtained from every patient at the time of enrollment.
Ethical Approval
Institutional Review Board approval was obtained (E2012036, bc2016039, and A2011-030-01).
Study subjects or cohorts overlap
The study subjects were collected from two prospective clinical trials (NCT01792999 and NCT03861221), aiming to assess the performance of new-generation breast imaging modality—CBBCT and explore the clinical application guideline of CBBCT, respectively. There have been several publications that share the same cohort with the current study regarding breast coverage and patient comfort comparison, diagnostic performance analysis, visual and quantitative breast density assessment, molecular subtyping, tumor size evaluation, and BI-RADS atlas exploration. In contrast, here we investigated axillary lymph node status and metastatic burden prediction that has not been evaluated previously.
• Li H, Yin L, Ye Z et al (2015) Comparative study of breast tissue coverage in cone-beam breast CT versus digital mammography. Chin J Radiol 49:488-490 in Chinese
• He N, Wu YP, Kong Y et al (2016) The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: a prospective study with 212 patients. Eur J Radiol 85:392-403
• Yin L, Ye Z (2016) New 3D X-ray modalities in breast imaging: digital breast tomosynthesis and cone-beam breast computed tomography. Chin Med Device Inform 22:17-20 in Chinese
• Liu A, Ye Z, Ma Y, Cao Y (2018) Reliability of breast density estimation based on cone-beam breast CT. Chin J Clin Oncol 45:246-250 in Chinese
• Liu A, Ma Y, Yin L, Han P, Li H, Ye Z (2018) Comparison of the diagnostic efficiency in breast malignancy between cone-beam breast CT and mammography in dense breast. Chin J Oncol 40:604-609 in Chinese
• Liu A, Ma Y, Yin L, Han P, Li H, Ye Z (2018) Diagnostic value of contrast-enhanced cone-beam breast CT in dense breast lesions. Chin Oncol 28:807-812 in Chinese
• Ma Y, Ye Z, Liu A, Yin L, Han P, Li H (2019) The accuracy of tumor size evaluation on invasive breast cancer based on cone-beam breast CT. Chin J Radiol 53:286-291 in Chinese
• Ma Y, Cao Y, Liu A et al (2019) A reliability comparison of cone-beam breast computed tomography and mammography: breast density assessment referring to the fifth edition of the BI-RADS atlas. Acad Radiol 26:752-759
• Li H, Yin L, He N et al (2019) Comparison of comfort between cone-beam breast computed tomography and digital mammography. Eur J Radiol 120:108674
• Zhu Y, Zhang Y, Ma Y et al (2020) Cone-beam breast CT features associated with HER2/neu overexpression in patients with primary breast cancer. Eur Radiol 30:2731-2739
• Ma Y, Liu A, O’Connell AM et al (2021) Contrast-enhanced cone-beam breast CT features of breast cancers: correlation with immunohistochemical receptors and molecular subtypes. Eur Radiol 31:2580-2589
• Wang Y, Ma Y, Zhu Y et al (2021) Value of cone-beam breast CT in differentiating benign from malignant dense breast masses. Chin J Radiol 55:961-967 in Chinese
• Zhang Y, Ma Y, Li Y et al (2021) Comparative study of cone-beam breast CT and breast MRI in diagnosis of BI-RADS 4 lesions on mammography or ultrasound. J Clin Radiol 40:1703-1708 in Chinese
• Zhu Y, O'Connell AM, Ma Y et al (2022) Dedicated breast CT: state of the art-part I. historical evolution and technical aspects. Eur Radiol 32:1579-1589
• Zhu Y, O'Connell AM, Ma Y et al (2022) Dedicated breast CT: state of the art-part II. clinical application and future outlook. Eur Radiol 32:2286-2300
• Liu A, Yin L, Ma Y et al (2022) Quantitative breast density measurement based on three-dimensional images: a study on cone-beam breast computed tomography. Acta Radiol 63:1023-1031
• Ma Y, Liu A, Zhang Y et al (2022) Comparison of background parenchymal enhancement (BPE) on contrast-enhanced cone-beam breast CT (CE-CBBCT) and breast MRI. Eur Radiol 32:5773-5782
• Liu A, Ma Y, Yin L et al (2023) Comparison of malignant calcification identification between breast cone-beam computed tomography and digital mammography. Acta Radiol 64:962-970
• Wang Y, Zhao M, Ma Y et al (2023) Accuracy of preoperative contrast-enhanced cone-beam breast CT in assessment of residual tumor after neoadjuvant chemotherapy: a comparative study with breast MRI. Acad Radiol 30:1805-1815
Methodology
-
Retrospective
-
diagnostic or prognostic study
-
multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 182 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Ma, Y., Zhai, Z. et al. Radiomics in cone-beam breast CT for the prediction of axillary lymph node metastasis in breast cancer: a multi-center multi-device study. Eur Radiol 34, 2576–2589 (2024). https://doi.org/10.1007/s00330-023-10256-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10256-4