Abstract
Objectives
To explore the potential of dynamic contrast-enhanced MRI (DCE-MRI) quantitative parameters in predicting severe acute radiation-induced rectal injury (RRI) in rectal cancer.
Methods
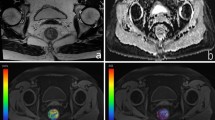
This retrospective study enrolled 49 patients with rectal cancer who underwent neoadjuvant chemoradiotherapy and rectal MRI including a DCE-MRI sequence from November 2014 to March 2021. Two radiologists independently measured DCE-MRI quantitative parameters, including the forward volume transfer constant (Ktrans), rate constant (kep), fractional extravascular extracellular space volume (ve), and the thickness of the rectal wall farthest away from the tumor. These parameters were compared between mild and severe acute RRI groups based on histopathological assessment. Receiver operating characteristic curve analysis was performed to analyze statistically significant parameters.
Results
Forty-nine patients (mean age, 54 years ± 12 [standard deviation]; 37 men) were enrolled, including 25 patients with severe acute RRI. Ktrans was lower in severe acute RRI group than mild acute RRI group (0.032 min−1 vs 0.054 min−1; p = 0.008), but difference of other parameters (kep, ve and rectal wall thickness) was not significant between these two groups (all p > 0.05). The area under the receiver operating characteristic curve of Ktrans was 0.72 (95% confidence interval: 0.57, 0.84). With a Ktrans cutoff value of 0.047 min−1, the sensitivity and specificity for severe acute RRI prediction were 80% and 54%, respectively.
Conclusion
Ktrans demonstrated moderate diagnostic performance in predicting severe acute RRI.
Clinical relevance statement
Dynamic contrast-enhanced MRI can provide non-invasive and objective evidence for perioperative management and treatment strategies in rectal cancer patients with acute radiation-induced rectal injury.
Key Points
• To our knowledge, this study is the first to evaluate the predictive value of contrast-enhanced MRI (DCE-MRI) quantitative parameters for severe acute radiation-induced rectal injury (RRI) in patients with rectal cancer.
• Forward volume transfer constant (Ktrans), derived from DCE-MRI, exhibited moderate diagnostic performance (AUC = 0.72) in predicting severe acute RRI of rectal cancer, with a sensitivity of 80% and specificity of 54%.
• DCE-MRI is a promising imaging marker for distinguishing the severity of acute RRI in patients with rectal cancer.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver operating characteristic curve
- CI:
-
Confidence interval
- DCE-MRI:
-
Dynamic contrast-enhanced MRI
- ICC:
-
Intraclass correlation coefficient
- k ep :
-
Rate constant
- K trans :
-
Forward volume transfer constant
- ROI:
-
Region of interest
- RRI:
-
Radiation-induced rectal injury
- v e :
-
Fractional extravascular extracellular space volume
- VOI:
-
Volume of interest
References
Benson AB, Venook AP, Al-Hawary MM et al (2020) NCCN Guidelines Insights: rectal cancer, Version 6.2020. J Natl Compr Canc Netw 18:806–815
Hauer-Jensen M, Denham JW, Andreyev HJ (2014) Radiation enteropathy–pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 11:470–479
Grodsky MB, Sidani SM (2015) Radiation proctopathy Clin Colon Rectal Surg 28:103–111
Qin Q, Zhu Y, Wu P et al (2019) Radiation-induced injury on surgical margins: a clue to anastomotic leakage after rectal-cancer resection with neoadjuvant chemoradiotherapy? Gastroenterol Rep (Oxf) 7:98–106
Espín E, Ciga MA, Pera M, Ortiz H (2015) Oncological outcome following anastomotic leak in rectal surgery. Br J Surg 102:416–422
Heemsbergen WD, Peeters ST, Koper PC, Hoogeman MS, Lebesque JV (2006) Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: consequential late damage. Int J Radiat Oncol Biol Phys 66:3–10
Dörr W, Hendry JH (2001) Consequential late effects in normal tissues. Radiother Oncol 61:223–231
Wu XR, Liu XL, Katz S, Shen B (2015) Pathogenesis, diagnosis, and management of ulcerative proctitis, chronic radiation proctopathy, and diversion proctitis. Inflamm Bowel Dis 21:703–715
Tabaja L, Sidani SM (2018) Management of radiation proctitis. Dig Dis Sci 63:2180–2188
Wu PH, Zhong QH, Ma TH et al (2020) To what extent should the intestinal be resected proximally after radiotherapy: hint from a pathological view. Gastroenterol Rep (Oxf) 8:277–285
Wachter S, Gerstner N, Goldner G, Pötzi R, Wambersie A, Pötter R (2000) Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother Oncol 54:11–19
Chrouser KL, Leibovich BC, Sweat SD et al (2005) Urinary fistulas following external radiation or permanent brachytherapy for the treatment of prostate cancer. J Urol 173:1953–1957
Addley HC, Vargas HA, Moyle PL, Crawford R, Sala E (2010) Pelvic imaging following chemotherapy and radiation therapy for gynecologic malignancies. Radiographics 30:1843–1856
Wu XM, Li YJ, Xie PY et al (2021) Predictive value of magnetic resonance imaging characteristics before and after radiotherapy for the occurrence of severe radiation-induced late rectal injury in patients with rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 24:991–997
Zappa M, Doblas S, Cazals-Hatem D et al (2018) Quantitative MRI in murine radiation-induced rectocolitis: comparison with histopathological inflammation score. NMR Biomed 31:e3897
Wu P, Li L, Wang H et al (2018) Role of angiogenesis in chronic radiation proctitis: new evidence favoring inhibition of angiogenesis ex vivo. Dig Dis Sci 63:113–125
Zhong QH, Wu PH, Qin QY et al (2017) Pathological insights of radiotherapy-related damage to surgical margin after preoperative radiotherapy in patients with rectal cancer. Zhonghua wai ke za zhi 55:507–514
Hasleton PS, Carr N, Schofield PF (1985) Vascular changes in radiation bowel disease. Histopathol 9:517–534
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Langberg CW, Waldron JA, Baker ML, Hauer-Jensen M (1994) Significance of overall treatment time for the development of radiation-induced intestinal complications. An experimental study in the rat. Cancer 73:2663–2668
Ota H, Takase K, Rikimaru H et al (2005) Quantitative vascular measurements in arterial occlusive disease. Radiographics 25:1141–1158
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Goh V, Padhani AR, Rasheed S (2007) Functional imaging of colorectal cancer angiogenesis. Lancet Oncol 8:245–255
Yao WW, Zhang H, Ding B et al (2011) Rectal cancer: 3D dynamic contrast-enhanced MRI; correlation with microvascular density and clinicopathological features. Radiol Med 116:366–374
Benjaminsen IC, Brurberg KG, Ruud EB, Rofstad EK (2008) Assessment of extravascular extracellular space fraction in human melanoma xenografts by DCE-MRI and kinetic modeling. Magn Reson Imaging 26:160–170
Blood CH, Zetter BR (1990) Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochem Biophys Acta 1032:89–118
Cao F, Ma TH, Liu GJ et al (2017) Correlation between disease activity and endorectal ultrasound findings of chronic radiation proctitis. Ultrasound Med Biol 43:2182–2191
Yang X, Chen Y, Wen Z et al (2019) Role of quantitative dynamic contrast-enhanced MRI in evaluating regional lymph nodes with a short-axis diameter of less than 5 mm in rectal cancer. AJR Am J Roentgenol 212:77–83
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370
Acknowledgements
We thank very much Yun-zhu Wu and Da Shi for their time and valuable contributions to the study.
Funding
This study has received funding by Guangdong Basic and Applied Basic Research Foundation (2020A1515010796).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shen-ping Yu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board (The First Affiliated Hospital, Sun Yat-sen University).
Ethical approval
Institutional Review Board (The First Affiliated Hospital, Sun Yat-sen University) approval was obtained.
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
-
Retrospective
-
Diagnostic or prognostic study
-
Performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Ding, L., Zhang, Zw. et al. Role of dynamic contrast-enhanced MRI in predicting severe acute radiation-induced rectal injury in patients with rectal cancer. Eur Radiol 34, 1471–1480 (2024). https://doi.org/10.1007/s00330-023-10194-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10194-1