Abstract
Objectives
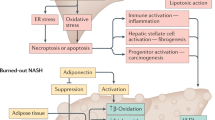
To evaluate the association between fat infiltration in skeletal muscles (myosteatosis) and hepatocellular carcinoma (HCC) in patients with non-alcoholic fatty liver disease (NAFLD).
Methods
In a cross-sectional cohort of 72 histologically proven NAFLD patients (n = 38 with non-alcoholic steatohepatitis; NASH), among which 20 had HCC diagnosed on biopsy, we used proton density fat fraction (PDFF) at MRI to evaluate myosteatosis in skeletal muscles (mean fat fraction and first order radiomic-based pattern) at the third lumbar level, namely in erector spinae (ES), quadratus lumborum (QL), psoas, oblique, and rectus muscles.
Results
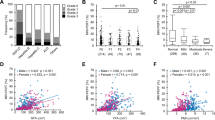
PDFFES was 70% higher in patients with HCC when compared to those without HCC (9.6 ± 5.5% versus 5.7 ± 3.0%, respectively, p < 0.001). In multivariate logistic regression, PDFFES was a significant predictor of the presence of HCC (AUC = 0.72, 95% CI 0.57–0.86, p = 0.002) independently from age, sex, visceral fat area, and liver fibrosis stage (all p < 0.05). The relationship between PDFFES and HCC was exacerbated in patients with NASH (AUC = 0.79, 95% CI 0.63–0.86, p = 0.006). In patients with NASH, radiomics features of heterogeneity such as energy and entropy in any of the paraspinal muscles (i.e., ES, QL, or psoas) were independent predictors of HCC. EnergyES identified patients with HCC (n = 13) in the NASH population with AUC = 0.92 (95% CI 0.82–1.00, p < 0.001).
Conclusion
In patients with NAFLD, and more specifically in those with NASH, the degree and heterogeneity of myosteatosis is independently associated with HCC irrespective of liver fibrosis stage.
Clinical relevance statement
Our data suggest that myosteatosis could be used as a biomarker of HCC in the ever-expanding NAFLD population and pave the way for further investigation in longitudinal studies.
Key Points
• HCC in patients with non-alcoholic fatty liver disease, and more specifically in those with non-alcoholic steatohepatitis, is independently associated with severe fatty infiltration (myosteatosis) of paravertebral skeletal muscles.
• Association between myosteatosis and HCC is independent from liver fibrosis stage.
• Histogram-based radiomics features of myosteatosis predicts the risk of HCC in patients with non-alcoholic steatohepatitis.


Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the receiver operating characteristic curve
- BMI:
-
Body mass index
- HCC:
-
Hepatocellular carcinoma
- MRI:
-
Magnetic resonance imaging
- NAFL:
-
Non-alcoholic fatty liver
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- PDFF:
-
Proton density fat fraction
- ROI:
-
Region of interest
References
Rumgay H, Arnold M, Ferlay J et al (2022) Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 77(6):1598–1606. https://doi.org/10.1016/j.jhep.2022.08.021
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin. doi:https://doi.org/10.3322/caac.20107.Available
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142(6):1264-1273.e1. https://doi.org/10.1053/j.gastro.2011.12.061
Sanyal AJ (2019) Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 16(6):377–386. https://doi.org/10.1038/s41575-019-0144-8
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ (2018) Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67(1):123–133. https://doi.org/10.1002/hep.29466
Huang DQ, El-Serag HB, Loomba R (2021) Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 18(4):223–238. https://doi.org/10.1038/s41575-020-00381-6
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84. https://doi.org/10.1002/hep.28431
Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M (2019) From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 16(7):411–428. https://doi.org/10.1038/s41575-019-0145-7
Nachit M, Leclercq IA (2019) Emerging awareness on the importance of skeletal muscle in liver diseases: time to dig deeper into mechanisms! Clin Sci 133(3):465–481. https://doi.org/10.1042/CS20180421
Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Malpica L, Williams GR (2020) Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 145:102839. https://doi.org/10.1016/j.critrevonc.2019.102839
Martin L, Birdsell L, MacDonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547. https://doi.org/10.1200/JCO.2012.45.2722
Hamaguchi Y, Kaido T, Okumura S et al (2016) Muscle steatosis is an independent predictor of postoperative complications in patients with hepatocellular carcinoma. World J Surg 40(8):1959–1968. https://doi.org/10.1007/s00268-016-3504-3
Fujiwara N, Nakagawa H, Kudo Y et al (2015) Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63(1):131–140. https://doi.org/10.1016/j.jhep.2015.02.031
Tachi Y, Kozuka A, Hirai T et al (2018) Skeletal muscle fat deposition is associated with hepatocellular carcinoma development in patients with chronic liver disease. Nutrition 54:83–88. https://doi.org/10.1016/j.nut.2018.03.011
Kitajima Y, Eguchi Y, Ishibashi E et al (2010) Age-related fat deposition in multifidus muscle could be a marker for nonalcoholic fatty liver disease. J Gastroenterol 45(2):218–224. https://doi.org/10.1007/s00535-009-0147-2
Kitajima Y, Hyogo H, Sumida Y et al (2013) Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol Aust 28(9):1507–1514. https://doi.org/10.1111/jgh.12227
Nachit M, Kwanten WJ, Thissen JP, et al (2021) Muscle fat content is strongly associated with NASH: a longitudinal study in patients with morbid obesity. J Hepatol. doi:https://doi.org/10.1016/j.jhep.2021.02.037
Nachit M, Lanthier N, Rodriguez J et al (2021) A dynamic association between myosteatosis and liver stiffness: results from a prospective interventional study in obese patients. JHEP Rep. 3(4):100323. https://doi.org/10.1016/j.jhepr.2021.100323
Hsieh YC, Joo SK, Koo BK et al (2023) Myosteatosis, but not sarcopenia, predisposes NAFLD subjects to early steatohepatitis and fibrosis progression. Clin Gastroenterol Hepatol. 21:388–397.e10. https://doi.org/10.1016/j.cgh.2022.01.020
Bedossa P, Poitou C, Veyrie N et al (2012) Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 56(5):1751–1759. https://doi.org/10.1002/hep.25889
Bedossa P, Burt AA, Gouw AHA et al (2014) Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. https://doi.org/10.1002/hep.27173
Wanless IR (1995) Terminology of nodular hepatocellular lesions. Hepatology 22(3):983–993. https://doi.org/10.1002/hep.1840220341
Loumaye A, de Barsy M, Nachit M et al (2015) Role of Activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab 100(5):2030–2038. https://doi.org/10.1210/jc.2014-4318
Loumaye A, de Barsy M, Nachit M et al (2017) Circulating activin A predicts survival in cancer patients. J Cachexia Sarcopenia Muscle 8(5):768–777. https://doi.org/10.1002/jcsm.12209
Bachmann R, Leonard D, Nachit M et al (2018) Comparison between abdominal fat measured by CT and anthropometric indices as prediction factors for mortality and morbidity after colorectal surgery. Acta Gastroenterol Belg 81(4):477–483
Keddar M, Muylle T, Carrie E et al (2020) Non-invasive quantification of fat deposits in skeletal muscle predicts cardiovascular outcome in kidney failure. Front Physiol 11:1–11. https://doi.org/10.3389/fphys.2020.00130
Nachit M, Lanthier N, Rodriguez J et al (2021) A dynamic association between myosteatosis and liver stiffness: results from a prospective interventional study in obese patients. JHEP Rep. 3(4):100323. https://doi.org/10.1016/j.jhepr.2021.100323
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. https://doi.org/10.1148/radiol.2020191145
Heckman KM, Otemuyiwa B, Chenevert TL et al (2019) Validation of a DIXON-based fat quantification technique for the measurement of visceral fat using a CT-based reference standard. Abdom Radiol (NY) 44(1):346–354. https://doi.org/10.1007/s00261-018-1678-x
Hu HH, Chen J, Shen W (2016) Segmentation and quantification of adipose tissue by magnetic resonance imaging. MAGMA 29(2):259–276. https://doi.org/10.1007/s10334-015-0498-z
Meister FA, Lurje G, Verhoeven S et al (2022) The role of sarcopenia and myosteatosis in short- and long-term outcomes following curative-intent surgery for hepatocellular carcinoma in a European cohort. Cancers (Basel) 14(3):720. https://doi.org/10.3390/cancers14030720
Chen BB, Liang PC, Shih TTF et al (2023) Sarcopenia and myosteatosis are associated with survival in patients receiving immunotherapy for advanced hepatocellular carcinoma. Eur Radiol 33(1):512–522. https://doi.org/10.1007/s00330-022-08980-4
Yi X, Fu Y, Long Q et al (2022) Myosteatosis can predict unfavorable outcomes in advanced hepatocellular carcinoma patients treated with hepatic artery infusion chemotherapy and anti-PD-1 immunotherapy. Front Oncol. 12:892192. https://doi.org/10.3389/fonc.2022.892192
Correa-de-Araujo R, Addison O, Miljkovic I et al (2020) Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol 11. doi:https://doi.org/10.3389/fphys.2020.00963
Ahn H, Kim DW, Ko Y et al (2021) Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev. 70:101398. https://doi.org/10.1016/j.arr.2021.101398
Amini B, Boyle SP, Boutin RD, Lenchik L (2019) Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci 74(10):1671–1678. https://doi.org/10.1093/gerona/glz034
Andreoli A, Garaci F, Cafarelli FP, Guglielmi G (2016) Body composition in clinical practice. Eur J Radiol 85(8):1461–1468. https://doi.org/10.1016/j.ejrad.2016.02.005
Montano-Loza AJ, Mazurak VC, Ebadi M et al (2018) Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology 67(3):914–923. https://doi.org/10.1002/hep.29578
Imai K, Takai K, Maeda T et al (2018) Increased visceral fat volume raises the risk for recurrence of hepatocellular carcinoma after curative treatment. Oncotarget 9(18):14058–14067. https://doi.org/10.18632/oncotarget.24500
Meza-Junco J, Montano-Loza AJ, Baracos VE et al (2013) Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. https://doi.org/10.1097/MCG.0b013e318293a825
Zwanenburg A, Vallières M, Abdalah MA et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295(2):328–338. https://doi.org/10.1148/radiol.2020191145
Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM (1997) Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat. 190(4):505–513. https://doi.org/10.1017/S0021878296001914
Van Loon LJC, Koopman R, Manders R, Van Der Weegen W, Van Kranenburg GP, Keizer HA (2004) Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab. doi:https://doi.org/10.1152/ajpendo.00464.2003
Rantanen J, Rissanen A, Kalimo H (1994) Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J 3(6):331–335. https://doi.org/10.1007/BF02200146
Burian E, Syväri J, Holzapfel C et al (2018) Gender-and age-related changes in trunk muscle composition using chemical shift encoding-based water–fat MRI. Nutrients. https://doi.org/10.3390/nu10121972
Kiefer LS, Fabian J, Rospleszcz S et al (2018) Assessment of the degree of abdominal myosteatosis by magnetic resonance imaging in subjects with diabetes, prediabetes and healthy controls from the general population. Eur J Radiol 105:261–268. https://doi.org/10.1016/j.ejrad.2018.06.023
Arbanas J, StarcevicKlasan G, Nikolic M, Jerkovic R, Miljanovic I, Malnar D (2009) Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat 215(6):636–641. https://doi.org/10.1111/j.1469-7580.2009.01155.x
Gumucio JP, Qasawa AH, Ferrara PJ et al (2019) Reduced mitochondrial lipid oxidation leads to fat accumulation in myosteatosis. FASEB J 33(7):7863–7881. https://doi.org/10.1096/fj.201802457RR
Benador IY, Veliova M, Mahdaviani K et al (2018) Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab 27(4):869-885.e6. https://doi.org/10.1016/j.cmet.2018.03.003
Gemmink A, Daemen S, Brouwers B et al (2018) Dissociation of intramyocellular lipid storage and insulin resistance in trained athletes and type 2 diabetes patients; involvement of perilipin 5? J Physiol 596(5):857–868. https://doi.org/10.1113/JP275182
Bosma M (2016) Lipid droplet dynamics in skeletal muscle. Exp Cell Res 340(2):180–186. https://doi.org/10.1016/j.yexcr.2015.10.023
Severinsen MCK, Pedersen BK (2020) Muscle–organ crosstalk: the emerging roles of myokines. Endocr Rev 41(4):594–609. https://doi.org/10.1210/endrev/bnaa016
Rome S, Forterre A, Mizgier ML, Bouzakri K (2019) Skeletal musclereleased extracellular vesicles: state of the art. Front Physiol 10.. doi:https://doi.org/10.3389/fphys.2019.00929
Whitham M, Parker BL, Friedrichsen M et al (2018) Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27(1):237-251.e4. https://doi.org/10.1016/j.cmet.2017.12.001
Arfianti A, Pok S, Barn V et al (2020) Exercise retards hepatocarcinogenesis in obese mice independently of weight control. J Hepatol 73(1):140–148. https://doi.org/10.1016/j.jhep.2020.02.006
Reig M, Gambato M, Man NK et al (2019) Should patients with NAFLD/NASH Be surveyed for HCC? Transplantation 103(1):39–44. https://doi.org/10.1097/TP.0000000000002361
Younes R, Bugianesi E (2018) Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol 68(2):326–334. https://doi.org/10.1016/j.jhep.2017.10.006
Funding
This work was supported by a Ph.D. fellowship from FRIA (FNRS, Belgium) (grant number 31618719 (to M.N.)), by a post-doctoral fellowship of Bpifrance (grant PSPC HECAM to A.A.)), and by an FNRS PDR grant (T.0141.19 (to I.A.L.)). The study was performed by a laboratory member of France Life Imaging network (grant ANR-11-INBS-0006).
Author information
Authors and Affiliations
Contributions
M.N. conceived and designed the study; M.D.B., VV, and BVB recruited the patients and carried out data collection in human studies. M.N. and AA analyzed and interpreted the data. VP analyzed liver biopsies. IL and BVB critically discussed the study design, data, and manuscript for scientific content. M.N., M.D.B., IL, and BVB wrote the manuscript, with contribution from all authors.
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Maxime Nachit.
Conflict of interest
The authors have no conflict of interest to disclose.
Statistic and biometry
The authors thank Lieven Desmet (Louvain Institute of Data Analysis and Modeling in economics and statistics, Louvain-la-Neuve, Belgium) for assistance for statistical analyses.
Informed consent
Informed consent was waived because of the retrospective nature of the study.
Ethical approval
This retrospective study (project no. 2017–030) obtained local IRB approval.
Study subjects or cohorts overlap
Some patients were reported in https://link.springer.com/article/10.1007/s00330-021-08261-6. The paper is unrelated to the present work.
Methodology
• retrospective study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nachit, M., Dioguardi Burgio, M., Abyzov, A. et al. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease is associated with heterogeneous pattern of fat infiltration in skeletal muscles. Eur Radiol 34, 1461–1470 (2024). https://doi.org/10.1007/s00330-023-10131-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10131-2