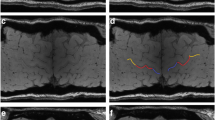
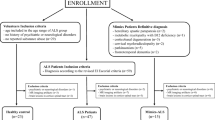
Abstract
Objective
The study aims at comparing the diagnostic accuracy of qualitative and quantitative assessment of the susceptibility in the precentral gyrus in detecting amyotrophic lateral sclerosis (ALS) with predominance of upper motor neuron (UMN) impairment.
Methods
We retrospectively collected clinical and 3T MRI data of 47 ALS patients, of whom 12 with UMN predominance (UMN-ALS). We further enrolled 23 healthy controls (HC) and 15 ALS Mimics (ALS-Mim). The Motor Cortex Susceptibility (MCS) score was qualitatively assessed on the susceptibility-weighted images (SWI) and automatic metrics were extracted from the quantitative susceptibility mapping (QSM) in the precentral gyrus. MCS scores and QSM-based metrics were tested for correlation, and ROC analyses.
Results
The correlation of MCS score and susceptibility skewness was significant (Rho = 0.55, p < 0.001). The susceptibility SD showed an AUC of 0.809 with a specificity and positive predictive value of 100% in differentiating ALS and ALS Mim versus HC, significantly higher than MCS (Z = −3.384, p-value = 0.00071). The susceptibility skewness value of −0.017 showed specificity of 92.3% and predictive positive value of 91.7% in differentiating UMN-ALS versus ALS mimics, even if the performance was not significantly better than MCS (Z = 0.81, p = 0.21).
Conclusion
The MCS and susceptibility skewness of the precentral gyrus show high diagnostic accuracy in differentiating UMN-ALS from ALS-mimics subjects. The quantitative assessment might be preferred being an automatic measure unbiased by the reader.
Clinical relevance statement
The clinical diagnostic evaluation of ALS patients might benefit from the qualitative and/or quantitative assessment of the susceptibility in the precentral gyrus as imaging marker of upper motor neuron predominance.
Key Points
• Amyotrophic lateral sclerosis diagnostic work-up lacks biomarkers able to identify upper motor neuron involvement.
• Susceptibility-weighted imaging/quantitative susceptibility mapping–based measures showed good diagnostic accuracy in discriminating amyotrophic lateral sclerosis with predominant upper motor neuron impairment from patients with suspected motor neuron disorder.
• Susceptibility-weighted imaging/quantitative susceptibility mapping–based assessment of the magnetic susceptibility provides a diagnostic marker for amyotrophic lateral sclerosis with upper motor neuron predominance.




Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
ALS Functional Rating Scale-Revised
- ALS-mim:
-
ALS-mimicking diseases
- c-ALS:
-
ALS patients with no clinically defined predominance
- HC:
-
Healthy controls
- LMN:
-
Lower motor neurons
- LMN-ALS:
-
LMN-predominant ALS
- MCS:
-
Motor Cortex Susceptibility
- QSM:
-
Quantitative susceptibility mapping
- SMD:
-
Suspected motor neuron disease
- SuscKurt:
-
Susceptibility kurtosis
- SuscMean:
-
Susceptibility mean
- SuscMedian:
-
Susceptibility median
- SuscSD:
-
Susceptibility standard deviation
- SuscSkew:
-
Susceptibility skewness
- SWI:
-
Susceptibility-weighted images
- UMN:
-
Upper motor neuron
- UMN-ALS:
-
UMN-predominant ALS
References
Brown RH Jr, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:1602
Lenglet T, Camdessanché J-P (2017) Amyotrophic lateral sclerosis or not: keys for the diagnosis. Rev Neurol 173:280–287
Mazón M, Vázquez Costa JF, Ten-Esteve A, Martí-Bonmatí L (2018) Imaging biomarkers for the diagnosis and prognosis of neurodegenerative diseases. The Example of Amyotrophic Lateral Sclerosis. Front Neurosci 12:784. https://doi.org/10.3389/fnins.2018.00784
Ludolph A, Drory V, Hardiman O et al (2015) A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Front Degener 16:291–292
Turner MR, Talbot K (2013) Mimics and chameleons in motor neurone disease. Pract Neurol 13:153–164
Acosta-Cabronero J, Machts J, Schreiber S et al (2018) Quantitative susceptibility MRI to detect brain iron in amyotrophic lateral sclerosis. Radiology 289:195–203
Conte G, Contarino VE, Casale S et al (2021) Amyotrophic lateral sclerosis phenotypes significantly differ in terms of magnetic susceptibility properties of the precentral cortex. Eur Radiol 31:5272–5280
Contarino VE, Conte G, Morelli C et al (2020) Toward a marker of upper motor neuron impairment in amyotrophic lateral sclerosis: a fully automatic investigation of the magnetic susceptibility in the precentral cortex. Eur J Radiol 124:108815
Dean KE, Shen B, Askin G et al (2021) A specific biomarker for amyotrophic lateral sclerosis: quantitative susceptibility mapping. Clin Imaging 75:125–130
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241
Adachi Y, Sato N, Saito Y et al (2015) Usefulness of SWI for the detection of iron in the motor cortex in amyotrophic lateral sclerosis. J Neuroimaging 25:443–451
Sheelakumari R, Madhusoodanan M, Radhakrishnan A et al (2016) A potential biomarker in amyotrophic lateral sclerosis: can assessment of brain iron deposition with SWI and corticospinal tract degeneration with DTI help? AJNR Am J Neuroradiol 37:252–258
Conte G, Sbaraini S, Morelli C et al (2021) A susceptibility-weighted imaging qualitative score of the motor cortex may be a useful tool for distinguishing clinical phenotypes in amyotrophic lateral sclerosis. Eur Radiol 31:1281–1289
Cosottini M, Donatelli G, Costagli M et al (2016) High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 37:455–461
Rizzo G, Marliani AF, Battaglia S, et al (2020) Diagnostic and prognostic value of conventional brain MRI in the clinical work-up of patients with amyotrophic lateral sclerosis. J Clin Med Res 9. https://doi.org/10.3390/jcm9082538
Eskreis-Winkler S, Zhang Y, Zhang J, et al (2017) The clinical utility of QSM: disease diagnosis, medical management, and surgical planning. NMR Biomed 30. https://doi.org/10.1002/nbm.3668
Quinn C, Edmundson C, Dahodwala N, Elman L (2020) Reliable and efficient scale to assess upper motor neuron disease burden in amyotrophic lateral sclerosis. Muscle Nerve 61:508–511
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Conte G, Sbaraini S, Morelli C et al (2021) Correction to: A susceptibility-weighted imaging qualitative score of the motor cortex may be a useful tool for distinguishing clinical phenotypes in amyotrophic lateral sclerosis. Eur Radiol 31:4404
Liu C, Li W, Tong KA et al (2015) Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging 42:23–41
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Sheelakumari R, Kesavadas C, Varghese T et al (2017) Assessment of iron deposition in the brain in frontotemporal dementia and its correlation with behavioral traits. AJNR Am J Neuroradiol 38:1953–1958
Yoshida M (2004) Amyotrophic lateral sclerosis with dementia: the clinicopathological spectrum. Neuropathology 24:87–102
Ferraro PM, Agosta F, Riva N et al (2017) Multimodal structural MRI in the diagnosis of motor neuron diseases. Neuroimage Clin 16:240–247
Kocar TD, Müller H-P, Ludolph AC, Kassubek J (2021) Feature selection from magnetic resonance imaging data in ALS: a systematic review. Ther Adv Chronic Dis 12:20406223211051000
Vázquez-Costa JF, Mazón M, Carreres-Polo J et al (2018) Brain signal intensity changes as biomarkers in amyotrophic lateral sclerosis. Acta Neurol Scand 137:262–271
Riku Y, Atsuta N, Yoshida M et al (2014) Differential motor neuron involvement in progressive muscular atrophy: a comparative study with amyotrophic lateral sclerosis. BMJ Open 4:e005213
Geser F, Stein B, Partain M et al (2011) Motor neuron disease clinically limited to the lower motor neuron is a diffuse TDP-43 proteinopathy. Acta Neuropathologica 121:509–517
Oba H, Araki T, Ohtomo K et al (1993) Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189:843–846
Dimov AV, Gillen KM, Nguyen TD et al (2022) Magnetic susceptibility source separation solely from gradient echo data: histological validation. Tomography 8:1544–1551
Acknowledgements
Authors would like to thank all the subjects that participated to the study.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Claudia Morelli.
Conflict of interest
Fabio Maria Triulzi is a member of the European Radiology Scientific Editorial Board. The remaining authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
The statistics were defined by Conte and Lo Russo
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
There exists a partial overlap with:
• Contarino VE, Conte G, Morelli C, et al (2020): Development of the QSM method, no SWI assessments were performed
• Conte G, Contarino VE, Casale S, et al (2021): Evaluation of the QSM measures accross the ALS clinical phenotypes, no SWI assessments were performed
• Conte G, Sbaraini S, Morelli C, et al (2021): Development of the SWI method, no QSM assessments were
Methodology
• Retrospective
• observational
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lo Russo , F., Contarino, V.E., Conte, G. et al. Amyotrophic lateral sclerosis with upper motor neuron predominance: diagnostic accuracy of qualitative and quantitative susceptibility metrics in the precentral gyrus. Eur Radiol 33, 7677–7685 (2023). https://doi.org/10.1007/s00330-023-10070-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-10070-y