Abstract
Objectives
To identify optimized MRI markers for evaluating chronic kidney disease (CKD) and renal interstitial fibrosis (IF).
Materials and methods
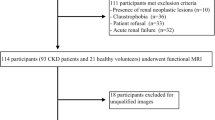
This prospective study included 43 patients with CKD and 20 controls. The CKD group was divided into mild and moderate-to-severe subgroups based on pathological results. Scanned sequences included T1 mapping, R2* mapping, intravoxel incoherent motion imaging, and diffusion-weighted imaging. One-way analyses of variance were used to compare MRI parameters among groups. Correlations of MRI parameters with estimated glomerular filtration rate (eGFR) and renal IF were analyzed using age as covariates. The support vector machine (SVM) model was used to evaluate the diagnostic efficacy of multiparametric MRI.
Results
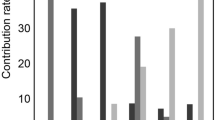
Compared to control values, renal cortical apparent diffusion coefficient (cADC), medullary ADC (mADC), cortical pure diffusion coefficient (cDt), medullary Dt (mDt), cortical shifted apparent diffusion coefficient (csADC), and medullary sADC (msADC) values gradually decreased in the mild and moderate-to-severe groups, while cortical T1 (cT1) and medullary T1 (mT1) values gradually increased. Values of cADC, mADC, cDt, mDt, cT1, mT1, csADC, and msADC were significantly associated with eGFR and IF (p < 0.001). The SVM model indicated that multiparametric MRI combining cT1 and csADC can distinguish patients with CKD from controls with high accuracy (0.84), sensitivity (0.70), and specificity (0.92) (AUC: 0.96). Multiparametric MRI combining cT1 and cADC exhibited high accuracy (0.91), sensitivity (0.95), and specificity (0.81) for evaluating IF severity (AUC: 0.96).
Conclusion
Multiparametric MRI combining T1 mapping and diffusion imaging may be of clinical utility in non-invasive assessment of CKD and IF.
Clinical relevance statement
This study shows that multiparametric MRI combining T1 mapping and diffusion imaging may be clinically useful in the non-invasive assessment of chronic kidney disease (CKD) and interstitial fibrosis; this could provide information for risk stratification, diagnosis, treatment, and prognosis.
Key Points
• Optimized MRI markers for evaluating chronic kidney disease and renal interstitial fibrosis were investigated.
• Renal cortex/medullary T1 values increased as interstitial fibrosis increased; cortical shifted apparent diffusion coefficient (csADC) correlated significantly with eGFR and interstitial fibrosis.
• Support vector machine (SVM) combining cortical T1 (cT1) and csADC/cADC effectively identifies chronic kidney disease and accurately predicts renal interstitial fibrosis.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- Dp:
-
Pseudo-diffusion coefficient
- Dt:
-
Pure diffusion coefficient
- DWI:
-
Diffusion-weighted imaging
- eGFR:
-
Estimated glomerular filtration rate
- Fp:
-
Perfusion score
- IF:
-
Interstitial fibrosis
- IVIM:
-
Intravoxel incoherent motion imaging
- sADC:
-
Shifted apparent diffusion coefficient
- T1:
-
Longitudinal relaxation time
References
Mariani LH, Martini S, Barisoni L et al (2018) Interstitial fibrosis scored on whole-slide digital imaging of kidney biopsies is a predictor of outcome in proteinuric glomerulopathies. Nephrol Dial Transplant 33:310–318
Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J (2005) Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl 99:S82–S86
Taal MW, Brenner BM (2016) Adaptation to nephron loss and mechanisms of progression in chronic kidney disease. Brenner & Rector’s the kidney, 10th edn. Elsevier, Philadelphia
Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK (2010) Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298:F1078–F1094
Berchtold L, Crowe LA, Friedli I et al (2020) Diffusion magnetic resonance imaging detects an increase in interstitial fibrosis earlier than the decline of renal function. Nephrol Dial Transplant 35:1274–1276
Quinn GZ, Abedini A, Liu H et al (2021) Renal histologic analysis provides complementary information to kidney function measurement for patients with early diabetic or hypertensive disease. J Am Soc Nephrol 32:2863–2876
Rush DN, Cockfield SM, Nickerson PW et al (2009) Factors associated with progression of interstitial fibrosis in renal transplant patients receiving tacrolimus and mycophenolate mofetil. Transplantation 88:897–903
Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD (2010) Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc of Nephrol 21:1987–1997
Barbour SJ, Espino-Hernandez G, Reich HN et al (2016) The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 89:167–175
Li Q, Li J, Zhang L, Chen Y, Zhang M, Yan F (2014) Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol 83:756–762
Zhao J, Wang ZJ, Liu M et al (2014) Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol 69:1117–1122
Wang W, Yu Y, Wen J et al (2019) Combination of functional magnetic resonance imaging and histopathologic analysis to evaluate interstitial fibrosis in kidney allografts. Clin J Am Soc Nephrol 14:1372–1380
Friedli I, Crowe LA, de Perrot T et al (2017) Comparison of readout-segmented and conventional single-shot for echo-planar diffusion-weighted imaging in the assessment of kidney interstitial fibrosis. J Magn Reson Imaging 46:1631–1640
Mao W, Zhou J, Zeng M et al (2018) Intravoxel incoherent motion diffusion-weighted imaging for the assessment of renal fibrosis of chronic kidney disease: a preliminary study. Magn Reson Imaging 47:118–124
Yu YM, Wang W, Wen J, Zhang Y, Lu GM, Zhang LJ (2021) Detection of renal allograft fibrosis with MRI: arterial spin labeling outperforms reduced field-of-view IVIM. Eur Radiol 31:6696–6707
Pruijm M, Milani B, Pivin E et al (2018) Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int 93:932–940
Hueper K, Peperhove M, Rong S et al (2014) T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur Radiol 24:2252–2260
Graham-Brown MP, Singh A, Wormleighton J et al (2019) Association between native T1 mapping of the kidney and renal fibrosis in patients with IgA nephropathy. BMC Nephrol 20:256
Azancot MA, Moreso F, Salcedo M et al (2014) The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int 85:1161–1168
Leung G, Kirpalani A, Szeto SG et al (2017) Could MRI be used to image kidney fibrosis? A review of recent advances and remaining barriers. Clin J Am Soc Nephrol 12:1019–1028
Zhang J, Yu Y, Liu X et al (2021) Evaluation of renal fibrosis by mapping histology and magnetic resonance imaging. Kidney Dis (Basel) 7:131–142
Selby NM, Blankestijn PJ, Boor P et al (2018) Magnetic resonance imaging biomarkers for chronic kidney disease: a position paper from the European Cooperation in Science and Technology Action PARENCHIMA. Nephrol Dial Transplant 33(suppl_2):ii4-ii14
Buchanan CE, Mahmoud H, Cox EF et al (2020) Quantitative assessment of renal structural and functional changes in chronic kidney disease using multi-parametric magnetic resonance imaging. Nephrol Dial Transplant 35:955–964
Levin A, Stevens PE, Bilous RW et al (2013) Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 1:1–150
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann of Intern Med 150:604–612
Iima M, Le Bihan D (2016) Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology 278:13–32
Le Bihan D, Ichikawa S, Motosugi U (2017) Diffusion and intravoxel incoherent motion MR imaging-based virtual elastography: a hypothesis-generating study in the liver. Radiology 285:609–619
Kromrey ML, Le Bihan D, Ichikawa S, Motosugi U (2020) Diffusion-weighted MRI-based virtual elastography for the assessment of liver fibrosis. Radiology 295:127–135
Yoshino N, Yamada I, Ohbayashi N et al (2001) Salivary glands and lesions: evaluation of apparent diffusion coefficients with split-echo diffusion-weighted MR imaging–initial results. Radiology 221:837–842
Chiaradia M, Baranes L, Van Nhieu JT et al (2014) Intravoxel incoherent motion (IVIM) MR imaging of colorectal liver metastases: are we only looking at tumor necrosis? J Magn Reson Imaging 39:317–325
Adams LC, Bressem KK, Scheibl S et al (2020) Multiparametric assessment of changes in renal tissue after kidney transplantation with quantitative MR relaxometry and diffusion-tensor imaging at 3 T. J Clin Med 9:1551
Berchtold L, Friedli I, Crowe LA et al (2020) Validation of the corticomedullary difference in magnetic resonance imaging-derived apparent diffusion coefficient for kidney fibrosis detection: a cross-sectional study. Nephrol Dial Transplant 35:937–945
Thoeny HC, Zumstein D, Simon-Zoula S et al (2006) Functional evaluation of transplanted kidneys with diffusion-weighted and Bold MR imaging: initial experience. Radiology 241:812–821
Bane O, Hectors SJ, Gordic S et al (2020) Multiparametric magnetic resonance imaging shows promising results to assess renal transplant dysfunction with fibrosis. Kidney Int 97:414–420
Yang Y, Zhang Z, Zhuo L, Chen DP, Li WG (2018) The spectrum of biopsy-proven glomerular disease in China: a systematic review. Chin Med J (Engl) 131(06):731–735
Liu ZH (2013) Nephrology in China. Nat Rev Nephrol 9:523–528
Pentang G, Lanzman RS, Heusch P et al (2014) Diffusion kurtosis imaging of the human kidney: a feasibility study. Magn Reson Imaging 32:413–420
Acknowledgements
We would like to express our gratitude to the participants and all the staff members for their cooperation to help finish the study.
Funding
This study has received funding by the National Natural Science Foundation of China (grant no. 81900698); Natural Science Foundation of Jiangsu Province (grant no. BK20210067); and Precision Medicine Key Project of Wuxi Health Commission (grant no. J202107).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Haoxiang Jiang.
Conflict of interest
One of the authors (Shaowei Hao) is an employee of Siemens Healthineers. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Study subjects or cohorts overlap
No study subjects or cohorts have been previously reported.
Methodology
-
prospective
-
diagnostic study/observational
-
performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenchen Hua, Lu Qiu, and Leting Zhou contributed equally to this work as co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hua, C., Qiu, L., Zhou, L. et al. Value of multiparametric magnetic resonance imaging for evaluating chronic kidney disease and renal fibrosis. Eur Radiol 33, 5211–5221 (2023). https://doi.org/10.1007/s00330-023-09674-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09674-1