Abstract
Objectives
The study investigated tumor burden dynamics on computed tomography (CT) scans in patients with advanced non-small-cell lung cancer (NSCLC) during first-line pembrolizumab plus chemotherapy, to provide imaging markers for overall survival (OS).
Methods
The study included 133 patients treated with first-line pembrolizumab plus platinum-doublet chemotherapy. Serial CT scans during therapy were assessed for tumor burden dynamics during therapy, which were studied for the association with OS.
Results
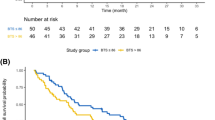
There were 67 responders, with overall response rate of 50%. The tumor burden change at the best overall response ranged from − 100.0% to + 132.1% (median of − 30%). Higher response rates were associated with younger age (p < 0.001) and higher programmed cell death-1 (PD-L1) expression levels (p = 0.01). Eighty-three patients (62%) showed tumor burden below the baseline burden throughout therapy. Using an 8-week landmark analysis, OS was longer in patients with tumor burden below the baseline burden in the first 8 weeks than in those who experienced ≥ 0% increase (median OS: 26.8 vs. 7.6 months, hazard ratio (HR): 0.36, p < 0.001). Tumor burden remained below their baseline throughout therapy was associated with significantly reduced hazards of death (HR: 0.72, p = 0.03) in the extended Cox models, after adjusting for other clinical variables. Pseudoprogression was noted in only one patient (0.8%).
Conclusions
Tumor burden staying below the baseline burden throughout the therapy was predictive of prolonged overall survival in patients with advanced NSCLC treated with first-line pembrolizumab plus chemotherapy, and may be used as a practical marker for therapeutic decisions in this widely used combination regimen.
Clinical relevance statement
The analysis of tumor burden dynamics on serial CT scans in reference to the baseline burden can provide an additional objective guide for treatment decision making in patients treated with first-line pembrolizumab plus chemotherapy for their advanced NSCLC.
Key Points
• Tumor burden remaining below baseline burden during therapy predicted longer survival during first-line pembrolizumab plus chemotherapy.
• Pseudoprogression was noted in 0.8%, demonstrating the rarity of the phenomenon.
• Tumor burden dynamics may serve as an objective marker for treatment benefit to guide treatment decisions during first-line pembrolizumab plus chemotherapy.





Similar content being viewed by others
Abbreviations
- BOR:
-
Best overall response
- CR:
-
Complete response
- CT:
-
Computed tomography
- CT:
-
Computed tomography
- ECOG:
-
Eastern Cooperative Oncology Group
- HR:
-
Hazard ratio
- ICI:
-
Immune-checkpoint inhibitors (ICI)
- MRI:
-
Magnetic resonance imaging
- NSCLC:
-
Non-small-cell lung cancer
- OS:
-
Overall survival
- PD:
-
Progressive disease
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death-ligand 1
- PET:
-
Positron emission tomography
- PR:
-
Partial response
- PS:
-
Performance status
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- TMB:
-
Tumor mutational burden
References
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Topalian SL, Sznol M, McDermott DF et al (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030
Nishino M, Tirumani SH, Ramaiya NH, Hodi FS (2015) Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 84:1259–1268
Reck M, Rodriguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. https://doi.org/10.1056/NEJMoa1606774
Langer CJ, Gadgeel SM, Borghaei H et al (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497–1508
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830
Park H, Sholl LM, Hatabu H, Awad MM, Nishino M (2019) Imaging of precision therapy for lung cancer: current state of the art. Radiology 293:15–29
Nishino M, Dahlberg SE, Adeni AE et al (2017) Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res 23:5737–5744
Nishino M, Hong F, Ricciuti B, Hatabu H, Awad MM (2021) Tumor response dynamics during first-line pembrolizumab therapy in patients with advanced non–small-cell lung cancer. JCO Precis Oncol. https://doi.org/10.1200/po.20.00478:501-509
Nishino M, Giobbie-Hurder A, Manos MP et al (2017) Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 23:4671–4679
Park HJ, Kim KW, Pyo J et al (2020) Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology 297:87–96
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD (2010) Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol 195:281–289
Nishino M, Cardarella S, Jackman DM et al (2013) RECIST 1.1 in NSCLC patients with EGFR mutations treated with EGFR tyrosine kinase inhibitors: comparison with RECIST 1.0. AJR Am J Roentgenol 201:W64-71
Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS (2013) Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 19:3936–3943
Nishino M, Jackman DM, Hatabu H et al (2010) New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 195:W221-228
Nishino M, Giobbie-Hurder A, Ramaiya NH, Hodi FS (2014) Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J Immunother Cancer 2:40
Nishino M, Ramaiya NH, Chambers ES et al (2016) Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer 4:84
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Hodi FS, Ballinger M, Lyons B et al (2018) Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 36:850–858
Seymour L, Bogaerts J, Perrone A et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152
Nishino M, Hatabu H, Hodi FS (2019) Imaging of cancer immunotherapy: current approaches and future directions. Radiology 290:9–22
Nishino M, Ramaiya NH, Hatabu H, Hodi FS (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14:655–668
Nishino M, Jagannathan JP, Krajewski KM et al (2012) Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 198:737–745
Lara PN Jr, Redman MW, Kelly K et al (2008) Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol 26:463–467
Gold KA, Kim ES, Lee JJ, Wistuba II, Farhangfar CJ, Hong WK (2011) The BATTLE to personalize lung cancer prevention through reverse migration. Cancer Prev Res (Phila) 4:962–972
Nishino M, Dahlberg SE, Cardarella S et al (2013) Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J Thorac Oncol 8:1059–1068
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092
Gettinger S, Rizvi NA, Chow LQ et al (2016) Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. https://doi.org/10.1200/jco.2016.66.9929
Camidge DR, Bang YJ, Kwak EL et al (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13:1011–1019
Oxnard GR, Morris MJ, Hodi FS et al (2012) When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst 104:1534–1541
Park K, Yu CJ, Kim SW et al (2016) First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION Study. JAMA Oncol 2:305–312
Ding T, Zhou F, Chen X et al (2017) Continuation of gefitinib plus chemotherapy prolongs progression-free survival in advanced non-small cell lung cancer patients who get acquired resistance to gefitinib without T790M mutations. J Thorac Dis 9:2923–2934
Nishino M (2018) Tumor response assessment for precision cancer therapy: response evaluation criteria in solid tumors and beyond. Am Soc Clin Oncol Educ Book. https://doi.org/10.1200/edbk_201441:1019-1029
Nishino M, Cardarella S, Dahlberg SE et al (2013) Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer 79:283–288
Funding
This study has received funding by R01CA203636 and U01CA209414.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mizuki Nishino, MD MPH.
Conflict of interest
Nishino: consultant to Daiichi Sankyo, AstraZeneca; research grant from Merck, Canon Medical Systems, AstraZeneca, Daiichi Sankyo.
Tseng, Alessi, Wang, Park, Vaz, Ricciuti, Lin, Christiani: None.
Hatabu: reserch funding from Canon Inc., Canon Medical Systems, and Konica-Minolta; consultant to Canon Medical Systems, and Mitsubishi Chemical Inc.
Awad: consultant/advisory board: Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, ArcherDX, Mirati, NextCure, Novartis, EMD Serono. Institutional research funding: from Bristol-Myers Squibb, AstraZeneca, Lilly, Genentech.
Statistics and biometry
Two of the authors have significant statistical expertise (Wang and Lin).
Informed consent
Written informed consent was obtained from all patients in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishino, M., Wang, X., Ricciuti, B. et al. Advanced non-small-cell lung cancer treated with first-line pembrolizumab plus chemotherapy: tumor response dynamics as a marker for survival. Eur Radiol 33, 7284–7293 (2023). https://doi.org/10.1007/s00330-023-09658-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09658-1