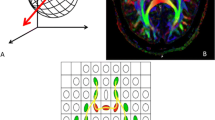
Abstract
Objectives
To investigate the recovery of human glymphatic system (GS) function in patients with temporal lobe epilepsy (TLE) after successful anterior temporal lobectomy (ATL) using diffusion tensor image analysis along the perivascular space (DTI-ALPS).
Methods
We retrospectively analysed DTI-ALPS index in 13 patients with unilateral TLE before and after ATL, and compared the index with 20 healthy controls (HCs). Two-sample t tests and paired t tests were performed to analyse differences in the DTI-ALPS index between patients and HCs. The Pearson correlation analysis was used to observe the relationship between the disease duration and GS function.
Results
The DTI-ALPS index before ATL was significantly lower in the hemisphere ipsilateral to the epileptogenic foci than in the contralateral hemisphere of the patients (p < 0.001, t = − 4.81) and in the ipsilateral hemisphere of the HCs (p = 0.007, t = − 2.90). A significant increase in the DTI-ALPS index was found in the hemisphere ipsilateral to the epileptogenic foci after successful ATL (p = 0.01, t = − 3.01). In addition, the DTI-ALPS index of the lesion side before ATL was significantly correlated with disease duration (p = 0.04, r = − 0.59).
Conclusions
DTI-ALPS may be used as a quantitative biomarker evaluating surgical outcomes and TLE disease duration. DTI-ALPS index may also help localise epileptogenic foci in unilateral TLE. Overall, our study suggests that GS may potentially serve as a new method for the management of TLE and a new direction for investigating the mechanism of epilepsy.
Key Points
• DTI-ALPS index may contribute to epileptogenic foci lateralisation in TLE.
• DTI-ALPS index is a potential quantitative feature evaluating surgical outcomes and TLE disease duration.
• The GS provides a new perspective for the study of TLE.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AQP4:
-
Aquaporin 4
- ATL:
-
Anterior temporal lobectomy
- Aβ:
-
Amyloid beta
- CSF:
-
Cerebrospinal fluid
- DTI:
-
Diffusion tensor imaging
- DTI-ALPS:
-
Diffusion tensor image analysis along the perivascular space
- FOV:
-
Field of view
- GS:
-
Glymphatic system
- HCs:
-
Healthy controls
- HS:
-
Hippocampal sclerosis
- ISF:
-
Interstitial fluid
- JME:
-
Juvenile myoclonic epilepsy
- MRI:
-
Magnetic resonance imaging
- PD:
-
Parkinson’s disease
- PVS:
-
Perivascular space
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SEEG:
-
Stereotactic electroencephalography
- TE:
-
Echo time
- TLE:
-
Temporal lobe epilepsy
- TR:
-
Repetition time
References
Bernhardt BC, Fadaie F, Liu M et al (2019) Temporal lobe epilepsy: hippocampal pathology modulates connectome topology and controllability. Neurology 92:e2209–e2220
Park BY, Larivière S, Rodríguez-Cruces R et al (2021) Topographic divergence of atypical cortical asymmetry and atrophy patterns in temporal lobe epilepsy. Brain. https://doi.org/10.1093/brain/awab417
De Tisi J, Bell GS, Peacock JL et al (2011) The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet 378:1388–1395
Engel J, Burchfiel J, Ebersole J et al (1993) Long-term monitoring for epilepsy. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 87:437–458
Iliff JJ, Wang M, Liao Y et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111
Tarasoff-Conway JM, Carare RO, Osorio RS et al (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11:457–470
Hablitz LM, Nedergaard M (2021) The glymphatic system: a novel component of fundamental neurobiology. J Neurosci 41:7698–7711
Iliff JJ, Chen MJ, Plog BA et al (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci 34:16180–16193
Lee DA, Park BS, Ko J et al (2022) Glymphatic system dysfunction in temporal lobe epilepsy patients with hippocampal sclerosis. Epilepsia Open. https://doi.org/10.1002/epi4.12594
Rangroo Thrane V, Thrane AS, Plog BA et al (2013) Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 3:2582
Zbesko JC, Nguyen TV, Yang T et al (2018) Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis 112:63–78
Taoka T, Masutani Y, Kawai H et al (2017) Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 35:172–178
Taoka T, Ito R, Nakamichi R et al (2022) Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol 40:147–158
Xie L, Kang H, Xu Q et al (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377
Ma X, Li S, Li C et al (2021) Diffusion tensor imaging along the perivascular space index in different stages of Parkinson’s disease. Front Aging Neurosci 13:773951
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024
Yang G, Deng N, Liu Y, Gu Y, Yao X (2020) Evaluation of glymphatic system using diffusion MR technique in T2DM cases. Front Hum Neurosci 14:300
Christensen J, Yamakawa GR, Shultz SR, Mychasiuk R (2021) Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog Neurobiol 198:101917
Barker-Haliski M, White HS (2015) Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med 5:a022863
Marchi N, Banjara M, Janigro D (2016) Blood-brain barrier, bulk flow, and interstitial clearance in epilepsy. J Neurosci Methods 260:118–124
Puvenna V, Engeler M, Banjara M et al (2016) Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res 1630:225–240
Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013) Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698:6–18
Lee HJ, Lee DA, Shin KJ, Park KM (2022) Glymphatic system dysfunction in patients with juvenile myoclonic epilepsy. J Neurol 269:2133–2139
Lee DA, Park BS, Ko J et al (2022) Glymphatic system function in patients with newly diagnosed focal epilepsy. Brain Behav 12:e2504
Liu C, Habib T, Salimeen M et al (2020) Quantification of visible Virchow-Robin spaces for detecting the functional status of the glymphatic system in children with newly diagnosed idiopathic generalized epilepsy. Seizure 78:12–17
Liu K, Zhu J, Chang Y et al (2021) Attenuation of cerebral edema facilitates recovery of glymphatic system function after status epilepticus. JCI Insight 6:e151835
Ahn JH, Cho H, Kim JH et al (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572:62–66
Louveau A, Smirnov I, Keyes TJ et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341
Iliff JJ, Wang M, Zeppenfeld DM et al (2013) Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 33:18190–18199
Demiral ŞB, Tomasi D, Sarlls J et al (2019) Apparent diffusion coefficient changes in human brain during sleep - does it inform on the existence of a glymphatic system? Neuroimage 185:263–273
Liu DX, He X, Wu D et al (2017) Continuous theta burst stimulation facilitates the clearance efficiency of the glymphatic pathway in a mouse model of sleep deprivation. Neurosci Lett 653:189–194
Sidhu MK, Duncan JS, Sander JW (2018) Neuroimaging in epilepsy. Curr Opin Neurol 31:371–378
Acknowledgements
The authors thank the patients who participated in this study and the staff of the Department of Radiology and Department of Functional Neurosurgery.
Funding
This work was supported by Doctor of Entrepreneurship and Innovation in Jiangsu Province (No. 2019204006), Research Project of Elderly Health in Jiangsu Province (No. LKM2023014) and Hospital-level Project of Affiliated Hospital of Xuzhou Medical University (No. 2020KA013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Kuncheng Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
The authors Chao Zhang and Kai Xu did statistical analyses.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Observational
• Performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, C., Xu, K., Zhang, H. et al. Recovery of glymphatic system function in patients with temporal lobe epilepsy after surgery. Eur Radiol 33, 6116–6123 (2023). https://doi.org/10.1007/s00330-023-09588-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09588-y