Abstract
Objectives
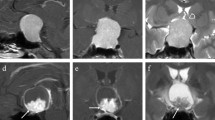
Pituitary adenomas can exhibit aggressive behavior, characterized by rapid growth, resistance to conventional treatment, and early recurrence. This study aims to evaluate the clinical value of shape-related features combined with textural features based on conventional MRI in evaluating the aggressiveness of pituitary adenomas and develop the best diagnostic model.
Methods
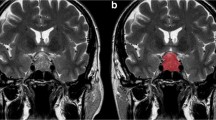
Two hundred forty-six pituitary adenoma patients (84 aggressive, 162 non-aggressive) who underwent preoperative MRI were retrospectively reviewed. The patients were divided into training (n = 193) and testing (n = 53) sets. Clinical information, shape-related, and textural features extracted from the tumor volume on contrast-enhanced T1-weighted images (CE-T1WI), were compared between aggressive and non-aggressive groups. Variables with significant differences were enrolled into Pearson’s correlation analysis to weaken multicollinearity. Logistic regression models based on the selected features were constructed to predict tumor aggressiveness under fivefold cross-validation.
Results
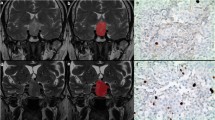
Sixty-five imaging features, including five shape-related and sixty textural features, were extracted from volumetric CE-T1WI. Forty-seven features were significantly different between aggressive and non-aggressive groups (all p values < 0.05). After feature selection, four features (SHAPE_Sphericity, SHAPE_Compacity, DISCRETIZED_Q3, and DISCRETIZED_Kurtosis) were put into logistic regression analysis. Based on the combination of these features and Knosp grade, the model yielded an area under the curve value of 0.935, with a sensitivity of 94.4% and a specificity of 82.9%, to discriminate between aggressive and non-aggressive pituitary adenomas in the testing set.
Conclusion
The radiomic model based on tumor shape and textural features study from CE-T1WI might potentially assist in the preoperative aggressiveness diagnosis of pituitary adenomas.
Key Points
• Pituitary adenomas with aggressive behavior exhibit rapid growth, resistance to conventional treatment, and early recurrence despite gross resection and may require multiline treatments.
• Shape-related features and texture features based on CE-T1WI were significantly correlated with the Ki-67 labeling index, mitotic count, and p53 expression, and the proposed model achieved a favorable prediction of the aggressiveness of PAs with an AUC value of 0.935.
• The prediction model might provide valuable guidance for individualized treatment in patients with PAs.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CE-T1WI:
-
Contrast-enhanced T1-weighted image
- ESE:
-
European Society of Endocrinology
- PAs:
-
Pituitary adenomas
- ROC:
-
Receiver operating characteristic
- WHO:
-
World Health Organization
References
Di Ieva A, Rotondo F, Syro LV, Cusimano MD, Kovacs K (2014) Aggressive pituitary adenomas—diagnosis and emerging treatments. Nat Rev Endocrinol. https://doi.org/10.1038/nrendo.2014.64
Dekkers OM, Karavitaki N, Pereira AM (2020) The epidemiology of aggressive pituitary tumors (and its challenges). Rev Endocr Metab Disord. https://doi.org/10.1007/s11154-020-09556-7
Raverot G, Burman P, McCormack A et al (2018) European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. https://doi.org/10.1530/eje-17-0796
Salehi F, Agur A, Scheithauer BW et al (2009) Ki-67 in pituitary neoplasms: a review–part I. Neurosurgery. https://doi.org/10.1227/01.Neu.0000349930.66434.82
Jaffrain-Rea ML, Di Stefano D, Minniti G et al (2002) A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: secreting versus non-secreting adenomas. Endocr Relat Cancer. https://doi.org/10.1677/erc.0.0090103
Gejman R, Swearingen B, Hedley-Whyte ET (2008) Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. https://doi.org/10.1016/j.humpath.2007.10.004
Hasanov R, Aydoğan B, Kiremitçi S, Erden E, Güllü S (2019) The prognostic roles of the Ki-67 proliferation index, P53 expression, mitotic index, and radiological tumor invasion in pituitary adenomas. Endocr Pathol. https://doi.org/10.1007/s12022-018-9563-2
Raverot G, Dantony E, Beauvy J et al (2017) Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2017-00773
Trouillas J, Roy P, Sturm N et al (2013) A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. https://doi.org/10.1007/s00401-013-1084-y
Raverot G, Ilie M, Lasolle H et al (2021) Aggressive pituitary tumours and pituitary carcinomas. Nat Rev Endocrinol. https://doi.org/10.1038/s41574-021-00550-w
Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW (2007) Patterns of contrast enhancement in the brain and meninges. Radiographics. https://doi.org/10.1148/rg.272065155
Ingrisch M, Schneider MJ, Nörenberg D et al (2017) Radiomic analysis reveals prognostic information in T1-weighted baseline magnetic resonance imaging in patients with glioblastoma. Invest Radiol. https://doi.org/10.1097/rli.0000000000000349
Danieli L, Riccitelli GC, Distefano D et al (2019) Brain tumor-enhancement visualization and morphometric assessment: a comparison of MPRAGE, SPACE, and VIBE MRI techniques. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A6096
Li N, Mo Y, Huang C et al (2021) A clinical semantic and radiomics nomogram for predicting brain invasion in WHO grade II meningioma based on tumor and tumor-to-brain interface features. Front Oncol. https://doi.org/10.3389/fonc.2021.752158
Galm BP, Martinez-Salazar EL, Swearingen B et al (2018) MRI texture analysis as a predictor of tumor recurrence or progression in patients with clinically non-functioning pituitary adenomas. Eur J Endocrinol. https://doi.org/10.1530/eje-18-0291
Ong T, Bharatha A, Alsufayan R, Das S, Lin AW (2021) MRI predictors for brain invasion in meningiomas. Neuroradiol J. https://doi.org/10.1177/1971400920953417
P L, E R-V, R L, et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. https://doi.org/10.1016/j.ejca.2011.11.036
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. https://doi.org/10.1038/nrclinonc.2017.141
Kniep HC, Madesta F, Schneider T et al (2019) Radiomics of brain MRI: utility in prediction of metastatic tumor type. Radiology. https://doi.org/10.1148/radiol.2018180946
Sun Q, Chen Y, Liang C, et al. Biologic pathways underlying prognostic radiomics phenotypes from paired MRI and RNA sequencing in glioblastoma. Radiology. doi:https://doi.org/10.1148/radiol.2021203281
Rui W, Qiao N, Wu Y et al (2022) Radiomics analysis allows for precise prediction of silent corticotroph adenoma among non-functioning pituitary adenomas. Eur Radiol. https://doi.org/10.1007/s00330-021-08361-3
Ugga L, Cuocolo R, Solari D et al (2019) Prediction of high proliferative index in pituitary macroadenomas using MRI-based radiomics and machine learning. Neuroradiology. https://doi.org/10.1007/s00234-019-02266-1
Niu J, Zhang S, Ma S et al (2019) Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images. Eur Radiol. https://doi.org/10.1007/s00330-018-5725-3
Li Y, Yan C, Weng S et al (2019) Texture analysis of multi-phase MRI images to detect expression of Ki67 in hepatocellular carcinoma. Clin Radiol. https://doi.org/10.1016/j.crad.2019.06.024
Blanchet L, Krooshof PWT, Postma GJ et al (2011) Discrimination between metastasis and glioblastoma multiforme based on morphometric analysis of MR images. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A2269
Cui Y, Tha KK, Terasaka S et al (2016) Prognostic imaging biomarkers in glioblastoma: development and independent validation on the basis of multiregion and quantitative analysis of MR images. Radiology. https://doi.org/10.1148/radiol.2015150358
Stoyanova R, Takhar M, Tschudi Y et al (2016) Prostate cancer radiomics and the promise of radiogenomics. Transl Cancer Res. https://doi.org/10.21037/tcr.2016.06.20
Yang G, Jones TL, Howe FA, Barrick TR (2016) Morphometric model for discrimination between glioblastoma multiforme and solitary metastasis using three-dimensional shape analysis. Magn Reson Med. https://doi.org/10.1002/mrm.25845
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. https://doi.org/10.1038/ncomms5006
Pérez-Beteta J, Molina-García D, Ortiz-Alhambra JA et al (2018) Tumor surface regularity at MR imaging predicts survival and response to surgery in patients with glioblastoma. Radiology. https://doi.org/10.1148/radiol.2018171051
Liu YQ, Gao BB, Dong B et al (2020) Preoperative vascular heterogeneity and aggressiveness assessment of pituitary macroadenoma based on dynamic contrast-enhanced MRI texture analysis. Eur J Radiol. https://doi.org/10.1016/j.ejrad.2020.109125
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. https://doi.org/10.1227/00006123-199310000-00008
Micko ASG, Wöhrer A, Höftberger R et al (2017) MGMT and MSH6 immunoexpression for functioning pituitary macroadenomas. Pituitary. https://doi.org/10.1007/s11102-017-0829-3
Nioche C, Orlhac F, Boughdad S et al (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. https://doi.org/10.1158/0008-5472.Can-18-0125
Zwanenburg A, Vallières M, Abdalah MA, et al. (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295:328–338
Sollini M, Cozzi L, Antunovic L, Chiti A, Kirienko M (2017) PET radiomics in NSCLC: state of the art and a proposal for harmonization of methodology. Sci Rep. https://doi.org/10.1038/s41598-017-00426-y
Deisboeck TS, Guiot C, Delsanto PP, Pugno N (2006) Does cancer growth depend on surface extension? Med Hypotheses 67:1338–1341
Caruana G, Pessini LM, Cannella R et al (2020) Texture analysis in susceptibility-weighted imaging may be useful to differentiate acute from chronic multiple sclerosis lesions. Eur Radiol. https://doi.org/10.1007/s00330-020-06995-3
Lopes MBS (2017) The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. https://doi.org/10.1007/s00401-017-1769-8
Madsen H, Borges TM, Knox AJ et al (2011) Giant pituitary adenomas: pathologic-radiographic correlations and lack of role for p53 and MIB-1 labeling. Am J Surg Pathol. https://doi.org/10.1097/PAS.0b013e31821e8c96
Batista GE, Prati RC, Monard MC (2004) A study of the behavior of several methods for balancing machine learning training data. ACM SIGKDD explorations newsletter.
Song Y, Zhang J, Zhang YD et al (2020) FeAture Explorer (FAE): a tool for developing and comparing radiomics models. PLoS One. https://doi.org/10.1371/journal.pone.0237587
George DH, Scheithauer BW, Kovacs K et al (2003) Crooke’s cell adenoma of the pituitary: an aggressive variant of corticotroph adenoma. Am J Surg Pathol. https://doi.org/10.1097/00000478-200310000-00005
Raverot G, Castinetti F, Jouanneau E et al (2012) Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clin Endocrinol. https://doi.org/10.1111/j.1365-2265.2012.04381.x
Conficoni A, Feraco P, Mazzatenta D et al (2020) Biomarkers of pituitary macroadenomas aggressive behaviour: a conventional MRI and DWI 3T study. Br J Radiol. https://doi.org/10.1259/bjr.20200321
Giesel FL, Mehndiratta A, Essig M (2010) High-relaxivity contrast-enhanced magnetic resonance neuroimaging: a review. Eur Radiol. https://doi.org/10.1007/s00330-010-1805-8
Lu CF, Hsu FT, Hsieh KL et al (2018) Machine Learning-Based Radiomics for Molecular Subtyping of Gliomas. Clin Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-17-3445
Zhou H, Vallières M, Bai HX et al (2017) MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro Oncol. https://doi.org/10.1093/neuonc/now256
Yang G, Jones TL, Barrick TR, Howe FA (2014) Discrimination between glioblastoma multiforme and solitary metastasis using morphological features derived from the p:q tensor decomposition of diffusion tensor imaging. NMR Biomed. https://doi.org/10.1002/nbm.3163
Berkmann S, Lattmann J, Schuetz P et al (2021) The Shape grading system: a classification for growth patterns of pituitary adenomas. Acta Neurochir. https://doi.org/10.1007/s00701-021-04912-1
Asano D, Kudo A, Akahoshi K et al (2020) Curative surgery and Ki-67 value rather than tumor differentiation predict the survival of patients with high-grade neuroendocrine neoplasms. Ann Surg. https://doi.org/10.1097/sla.0000000000004495
Sav A, Rotondo F, Syro LV, Scheithauer BW, Kovacs K (2012) Biomarkers of pituitary neoplasms. Anticancer Res 32:4639–4654
Frieboes HB, Zheng X, Sun CH et al (2006) An integrated computational/experimental model of tumor invasion. Cancer Res. https://doi.org/10.1158/0008-5472.Can-05-3166
Zou H (2013) Variable selection. Editorial. Stat Methods Med Res. https://doi.org/10.1177/0962280213506395
Cozzi L, Dinapoli N, Fogliata A et al (2017) Radiomics based analysis to predict local control and survival in hepatocellular carcinoma patients treated with volumetric modulated arc therapy. BMC Cancer. https://doi.org/10.1186/s12885-017-3847-7
Lam KY (2017) Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocrine pathology.
Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J (1997) Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocrinol Metab. https://doi.org/10.1210/jcem.82.7.4088
Obari A, Sano T, Ohyama K et al (2008) Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol. https://doi.org/10.1007/s12022-008-9029-z
Funding
This study has received funding by the National Natural Science Foundation of China (grant no.82171885), Shanghai Science and Technology Committee Project (Natural Science Funding; grant no.20ZR1433200), and Shanghai Science and Technology Committee Project (the Explorer Project Funding; grant no. 21TS1400700).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yan Zhou.
Conflict of interest
The authors (Yongming Dai and Hai Lin) of this manuscript declare relationships with the following companies: United Imaging Healthcare. The remaining authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• case–control study
• performed at one institution
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Dai, Y., Lin, H. et al. Shape and texture analyses based on conventional MRI for the preoperative prediction of the aggressiveness of pituitary adenomas. Eur Radiol 33, 3312–3321 (2023). https://doi.org/10.1007/s00330-023-09412-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-023-09412-7