Abstract
Objectives
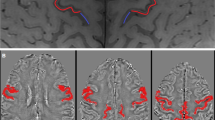
To explore whether the combined analysis of motor and bulbar region of M1 on susceptibility-weighted imaging (SWI) can be a valid biomarker for amyotrophic lateral sclerosis (ALS).
Methods
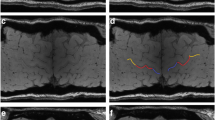
Thirty-two non-demented ALS patients and 35 age- and gender-matched healthy controls (HC) were retrospectively recruited. SWI and 3D-T1-MPRAGE images were obtained from all individuals using a 3.0-T MRI scan. The bilateral posterior band of M1 was manually delineated by three neuroradiologists on phase images and subdivided into the motor and bulbar regions. We compared the phase values in two groups and performed a stratification analysis (ALSFRS-R score, duration, disease progression rate, and onset). Receiver operating characteristic (ROC) curves were also constructed.
Results
ALS group showed significantly increased phase values in M1 and the two subregions than the HC group, on the all and elderly level (p < 0.001, respectively). On all-age level comparison, negative correlations were found between phase values of M1 and clinical score and duration (p < 0.05, respectively). Similar associations were found in the motor region (p < 0.05, respectively). On both the total (p < 0.01) and elderly (p < 0.05) levels, there were positive relationships between disease progression rate and M1 phase values. In comparing ROC curves, the entire M1 showed the best diagnostic performance.
Conclusions
Combining motor and bulbar analyses as an integral M1 region on SWI can improve ALS diagnosis performance, especially in the elderly. The phase value could be a valuable biomarker for ALS evaluation.
Key Points
• Integrated analysis of the motor and bulbar as an entire M1 region on SWI can improve the diagnosis performance in ALS.
• Quantitative analysis of iron deposition by SWI measurement helps the clinical evaluation, especially for the elderly patients.
• Phase value, when combined with the disease progression rate, could be a valuable biomarker for ALS.






Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
Revised ALS functional rating scale
- HC:
-
Healthy controls
- ICC:
-
Interclass correlation coefficient
- LMN:
-
Lower motor neurons
- M1:
-
Primary motor cortex
- MPRAGE:
-
Magnetization prepared rapid acquisition gradient echo
- QSM:
-
Quantitative susceptibility mapping
- SWI:
-
Susceptibility weighted imaging
- UMN:
-
Upper motor neurons
References
Brunet A, Stuart-Lopez G, Burg T et al (2020) Cortical circuit dysfunction as a potential driver of amyotrophic lateral sclerosis. Front Neurosci 14:363. https://doi.org/10.3389/fnins.2020.00363
Kiernan MC, Vucic S, Talbot K et al (2021) Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol 17:104–118. https://doi.org/10.1038/s41582-020-00434-z
Longinetti E, Fang F (2019) Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol 32:771–776. https://doi.org/10.1097/WCO.0000000000000730
Kiernan MC, Vucic S, Cheah BC et al (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955. https://doi.org/10.1016/s0140-6736(10)61156-7
van Es MA, Hardiman O, Chio A et al (2017) Amyotrophic lateral sclerosis. Lancet 390:2084–2098. https://doi.org/10.1016/S0140-6736(17)31287-4
Hadzhieva M, Kirches E, Wilisch-Neumann A et al (2013) Dysregulation of iron protein expression in the G93A model of amyotrophic lateral sclerosis. Neuroscience 230:94–101. https://doi.org/10.1016/j.neuroscience.2012.11.021
Kwan JY, Jeong SY, Van Gelderen P et al (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241. https://doi.org/10.1371/journal.pone.0035241
Petillon C, Hergesheimer R, Puy H et al (2019) The relevancy of data regarding the metabolism of iron to our understanding of deregulated mechanisms in ALS; hypotheses and pitfalls. Front Neurosci 12:1031. https://doi.org/10.3389/fnins.2018.01031
Moreau C, Danel V, Devedjian JC et al (2018) Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? Antioxid Redox Signal 29:742–748. https://doi.org/10.1089/ars.2017.7493
Oba H, Araki T, Ohtomo K et al (1993) Amyotrophic lateral sclerosis: T2 shortening in motor cortex at MR imaging. Radiology 189:843–846. https://doi.org/10.1148/radiology.189.3.8234713
Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A et al (2016) In vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci 36:364–374. https://doi.org/10.1523/JNEUROSCI.1907-15.2016
Bhattarai A, Chen Z, Ward PGD et al (2020) Serial assessment of iron in the motor cortex in limb-onset amyotrophic lateral sclerosis using quantitative susceptibility mapping. Quant Imaging Med Surg 10:1465–1476. https://doi.org/10.21037/qims-20-187
Haacke EM, Xu Y, Cheng YC, Reichenbach JR (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618. https://doi.org/10.1002/mrm.20198
Bhattarai A, Egan GF, Talman P et al (2021) Magnetic resonance iron imaging in amyotrophic lateral sclerosis. J Magn Reson Imaging 55:1283–1300. https://doi.org/10.1002/jmri.27530
Kee Y, Liu Z, Zhou L et al (2017) Quantitative susceptibility mapping (QSM) algorithms: mathematical rationale and computational implementations. IEEE Trans Biomed Eng 64:2531–2545. https://doi.org/10.1109/TBME.2017.2749298
Sheelakumari R, Madhusoodanan M, Radhakrishnan A et al (2015) A potential biomarker in amyotrophic lateral sclerosis: can assessment of brain iron deposition with SWI and corticospinal tract degeneration with DTI help? AJNR Am J Neuroradiol 37:252–258. https://doi.org/10.3174/ajnr.A4524
Haacke EM, Ayaz M, Khan A et al (2007) Establishing a baseline phase behavior in magnetic resonance imaging to determine normal vs. abnormal iron content in the brain. J Magn Reson Imaging 26:256–264. https://doi.org/10.1002/jmri.22987
Roeben B, Wilke C, Bender B et al (2019) The motor band sign in ALS: presentations and frequencies in a consecutive series of ALS patients. J Neurol Sci 406:116440. https://doi.org/10.1016/j.jns.2019.116440
Nnah I, Wessling-Resnick M (2018) Brain iron homeostasis: a focus on microglial iron. Pharmaceuticals 11:129. https://doi.org/10.3390/ph11040129
Adachi Y, Sato N, Saito Y et al (2015) Usefulness of SWI for the detection of iron in the motor cortex in amyotrophic lateral sclerosis. J Neuroimaging 25:443–451. https://doi.org/10.1111/jon.12127
Acosta-Cabronero J, Machts J, Schreiber S et al (2018) Quantitative susceptibility MRI to detect brain iron in amyotrophic lateral sclerosis. Radiology 289:195–203. https://doi.org/10.1148/radiol.2018180112
Wang C, Foxley S, Ansorge O et al (2020) Methods for quantitative susceptibility and R2* mapping in whole post-mortem brains at 7T applied to amyotrophic lateral sclerosis. Neuroimage 222:117216. https://doi.org/10.1016/j.neuroimage.2020.117216
Conte G, Sbaraini S, Morelli C et al (2021) A susceptibility-weighted imaging qualitative score of the motor cortex may be a useful tool for distinguishing clinical phenotypes in amyotrophic lateral sclerosis. Eur Radiol 31:1281–1289. https://doi.org/10.1007/s00330-020-07239-0
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. https://doi.org/10.1080/146608200300079536
Lobel U, Sedlacik J, Sabin ND et al (2010) Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology 52:1167–1177. https://doi.org/10.1007/s00234-010-0771-9
Cedarbaum JM, Stambler N, Malta E et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169:13–21. https://doi.org/10.1016/S0022-510X(99)00210-5
van der Burgh HK, Westeneng HJ, Walhout R et al (2020) Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology 94:e2592–e2604. https://doi.org/10.1212/WNL.0000000000009498
Turner MR, Barnwell J, Al-Chalabi A, Eisen A (2012) Young-onset amyotrophic lateral sclerosis: historical and other observations. Brain 135:2883–2891. https://doi.org/10.1093/brain/aws144
Kalra S, Müller H-P, Ishaque A et al (2020) A prospective harmonized multicenter DTI study of cerebral white matter degeneration in ALS. Neurology 95:e943–e952. https://doi.org/10.1212/WNL.0000000000010235
Geyer S, Weiss M, Reimann K et al (2011) Microstructural parcellation of the human cerebral cortex – from brodmann’s post-mortem map to in vivo mapping with high-field magnetic resonance imaging. Front Hum Neurosci 5:19. https://doi.org/10.3389/fnhum.2011.00019
Duyn JH, van Gelderen P, Li TQ et al (2007) High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A 104:11796–11801. https://doi.org/10.1073/pnas.0610821104
Cosottini M, Donatelli G, Costagli M et al (2016) High-resolution 7T MR imaging of the motor cortex in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 37:455–461. https://doi.org/10.3174/ajnr.A4562
Butman JA, Floeter MK (2007) Decreased thickness of primary motor cortex in primary lateral sclerosis. AJNR Am J Neuroradiol 28:87–91
Costagli M, Donatelli G, Biagi L et al (2016) Magnetic susceptibility in the deep layers of the primary motor cortex in amyotrophic lateral sclerosis. Neuroimage Clin 12:965–969. https://doi.org/10.1016/j.nicl.2016.04.011
Schweitzer AD, Liu T, Gupta A et al (2015) Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. AJR Am J Roentgenol 204:1086–1092. https://doi.org/10.2214/AJR.14.13459
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Leigh PN, Abrahams S, Al-Chalabi A et al (2003) The management of motor neurone disease. J Neurol Neurosurg Psychiatry 74(Suppl 4):iv32–iv47. https://doi.org/10.1136/jnnp.74.suppl_4.iv32
Kühnlein P, Gdynia HJ, Sperfeld AD et al (2008) Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol 4:366–374. https://doi.org/10.1038/ncpneuro0853
Yu J, Qi F, Wang N et al (2014) Increased iron level in motor cortex of amyotrophic lateral sclerosis patients: an in vivo MR study. Amyotroph Lateral Scler Frontotemporal Degener 15:357–361. https://doi.org/10.3109/21678421.2014.906618
Donatelli G, Caldarazzo Ienco E, Costagli M et al (2019) MRI cortical feature of bulbar impairment in patients with amyotrophic lateral sclerosis. Neuroimage Clin 24:101934. https://doi.org/10.1016/j.nicl.2019.101934
Zecca L, Youdim MBH, Riederer P et al (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5:863–873. https://doi.org/10.1038/nrn1537
Dean KE, Shen B, Askin G et al (2021) A specific biomarker for amyotrophic lateral sclerosis: Quantitative susceptibility mapping. Clin Imaging 75:125–130. https://doi.org/10.1016/j.clinimag.2020.12.018
Endo H, Sekiguchi K, Shimada H et al (2018) Low signal intensity in motor cortex on susceptibility-weighted MR imaging is correlated with clinical signs of amyotrophic lateral sclerosis: a pilot study. J Neurol 265:552–561. https://doi.org/10.1007/s00415-017-8728-0
Canna A, Trojsi F, Di Nardo F et al (2021) Combining structural and metabolic markers in a quantitative MRI study of motor neuron diseases. Ann Clin Transl Neurol 8:1774–1785. https://doi.org/10.1002/acn3.51418
Logroscino G, Traynor BJ, Hardiman O et al (2008) Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry 79:6–11. https://doi.org/10.1136/jnnp.2006.104828
Paydarnia P, Mayeli M, Shafie M et al (2021) Alterations of the serum and CSF ferritin levels and the diagnosis and prognosis of amyotrophic lateral sclerosis. eNeurologicalSci 25:100379. https://doi.org/10.1016/j.ensci.2021.100379
Lu C-H, Allen K, Oei F et al (2016) Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 3:e244. https://doi.org/10.1212/NXI.0000000000000244
Rooney J, Burke T, Vajda A et al (2017) What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 88:381–385. https://doi.org/10.1136/jnnp-2016-314661
Acknowledgements
The authors sincerely thank all the ALS patients and the healthy volunteers included in this study.
Funding
This work was supported by the National Key R&D Program of China (2019YFC0120602), the National Natural Science Foundation of China (61672236), the Science and Technology Commission of Shanghai Municipality (19ZR1407900), and the Scientific Research project of Huashan Hospital, Fudan University (2016QD15).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yuxin Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 9173 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bao, Y., Chen, Y., Piao, S. et al. Iron quantitative analysis of motor combined with bulbar region in M1 cortex may improve diagnosis performance in ALS. Eur Radiol 33, 1132–1142 (2023). https://doi.org/10.1007/s00330-022-09045-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09045-2