Abstract
Objectives
Develop and evaluate a deep learning–based automatic meningioma segmentation method for preoperative meningioma differentiation using radiomic features.
Methods
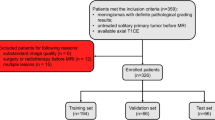
A retrospective multicentre inclusion of MR examinations (T1/T2-weighted and contrast-enhanced T1-weighted imaging) was conducted. Data from centre 1 were allocated to training (n = 307, age = 50.94 ± 11.51) and internal testing (n = 238, age = 50.70 ± 12.72) cohorts, and data from centre 2 external testing cohort (n = 64, age = 48.45 ± 13.59). A modified attention U-Net was trained for meningioma segmentation. Segmentation accuracy was evaluated by five quantitative metrics. The agreement between radiomic features from manual and automatic segmentations was assessed using intra class correlation coefficient (ICC). After univariate and minimum-redundancy-maximum-relevance feature selection, L1-regularized logistic regression models for differentiating between low-grade (I) and high-grade (II and III) meningiomas were separately constructed using manual and automatic segmentations; their performances were evaluated using ROC analysis.
Results
Dice of meningioma segmentation for the internal testing cohort were 0.94 ± 0.04 and 0.91 ± 0.05 for tumour volumes in contrast-enhanced T1-weighted and T2-weighted images, respectively; those for the external testing cohort were 0.90 ± 0.07 and 0.88 ± 0.07. Features extracted using manual and automatic segmentations agreed well, for both the internal (ICC = 0.94, interquartile range: 0.88–0.97) and external (ICC = 0.90, interquartile range: 0.78–70.96) testing cohorts. AUC of radiomic model with automatic segmentation was comparable with that of the model with manual segmentation for both the internal (0.95 vs. 0.93, p = 0.176) and external (0.88 vs. 0.91, p = 0.419) testing cohorts.
Conclusions
The developed deep learning–based segmentation method enables automatic and accurate extraction of meningioma from multiparametric MR images and can help deploy radiomics for preoperative meningioma differentiation in clinical practice.
Key Points
• A deep learning–based method was developed for automatic segmentation of meningioma from multiparametric MR images.
• The automatic segmentation method enabled accurate extraction of meningiomas and yielded radiomic features that were highly consistent with those that were obtained using manual segmentation.
• High-grade meningiomas were preoperatively differentiated from low-grade meningiomas using a radiomic model constructed on features from automatic segmentation.






Similar content being viewed by others
Abbreviations
- ASSD:
-
Average symmetric surface distance
- AUC:
-
Area under the curve
- CE-T1WI:
-
Contrast-enhanced T1-weighted imaging
- CETV:
-
Tumour volume segmented in CE-T1WI
- CNN:
-
Convolutional neural network
- HD95:
-
95% Hausdorff distance
- ICC:
-
Intraclass correlation coefficient
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- T1WI:
-
T1-weighted imaging
- T2TV:
-
Tumour volume segmented in T2WI
- T2WI:
-
T2-weighted imaging
- VOE:
-
Volume overlap error
References
Achey RL, Gittleman H, Schroer J, Khanna V, Kruchko C, Barnholtz-Sloan JS (2019) Nonmalignant and malignant meningioma incidence and survival in the elderly, 2005-2015, using the Central Brain Tumor Registry of the United States. Neuro Oncol 21:380–391
Whittle IR, Smith C, Navoo P, Collie D (2004) Meningiomas. Lancet 363:1535–1543
Chamoun R, Krisht KM, Couldwell WT (2011) Incidental meningiomas. Neurosurg Foc 31:E19
Li X, Miao Y, Han L et al (2019) Meningioma grading using conventional MRI histogram analysis based on 3D tumor measurement. Eur J Radiol 110:45–53
Modha A, Gutin PH (2005) Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 57:538–550 discussion 538-550
Goldbrunner R, Minniti G, Preusser M et al (2016) EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 17:e383–e391
Weber DC, Ares C, Villa S et al (2018) Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother Oncol 128:260–265
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Park YW, Oh J, You SC et al (2019) Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur Radiol 29:4068–4076
Laukamp KR, Shakirin G, Baeßler B et al (2019) Accuracy of radiomics-based feature analysis on multiparametric magnetic resonance images for noninvasive meningioma grading. World Neurosurg 132:e366–e390
Li X, Lu Y, Xiong J et al (2019) Presurgical differentiation between malignant haemangiopericytoma and angiomatous meningioma by a radiomics approach based on texture analysis. J Neuroradiol 46:281–287
Lu Y, Liu L, Luan S, Xiong J, Geng D, Yin B (2019) The diagnostic value of texture analysis in predicting WHO grades of meningiomas based on ADC maps: an attempt using decision tree and decision forest. Eur Radiol 29:1318–1328
Ke C, Chen H, Lv X et al (2020) Differentiation between benign and nonbenign meningiomas by using texture analysis from multiparametric MRI. J Magn Reson Imaging 51:1810–1820
Fiez JA, Damasio H, Grabowski TJ (2000) Lesion segmentation and manual warping to a reference brain: intra- and interobserver reliability. Hum Brain Mapp 9:192–211
Tsai YF, Chiang IJ, Lee YC, Liao CC, Wang KL (2005) Automatic MRI meningioma segmentation using estimation maximization. Conf Proc IEEE Eng Med Biol Soc 2005:3074–3077
Hsieh TM, Liu YM, Liao CC, Xiao F, Chiang IJ, Wong JM (2011) Automatic segmentation of meningioma from non-contrasted brain MRI integrating fuzzy clustering and region growing. BMC Med Inform Decis Mak 11:54
Latini F, Larsson EM, Ryttlefors M (2017) Rapid and accurate MRI segmentation of peritumoral brain edema in meningiomas. Clin Neuroradiol 27:145–152
Fu Y, Lei Y, Wang T, Curran WJ, Liu T, Yang X (2021) A review of deep learning based methods for medical image multi-organ segmentation. Phys Med 85:107–122
Zegers CML, Posch J, Traverso A et al (2021) Current applications of deep-learning in neuro-oncological MRI. Phys Med 83:161–173
Laukamp KR, Thiele F, Shakirin G et al (2019) Fully automated detection and segmentation of meningiomas using deep learning on routine multiparametric MRI. Eur Radiol 29:124–132
Laukamp KR, Pennig L, Thiele F et al (2021) Automated meningioma segmentation in multiparametric MRI : comparable effectiveness of a deep learning model and manual segmentation. Clin Neuroradiol 31:357–366
Zhang H, Mo J, Jiang H et al (2021) Deep learning model for the automated detection and histopathological prediction of meningioma. Neuroinformatics 19:393–402
Chen C, Cheng Y, Xu J et al (2021) Automatic meningioma segmentation and grading prediction: a hybrid deep-learning method. J Pers Med 11:786
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
Ronneberger O, Fischer P, Brox T (2015) U-Net: Convolutional networks for biomedical image segmentation. Springer International Publishing, Cham, pp 234–241
Zhang H, Wu C, Zhang Z et al (2020) ResNeSt: split-attention networks
Zhu Y, Man C, Gong L et al (2019) A deep learning radiomics model for preoperative grading in meningioma. Eur J Radiol 116:128–134
Banzato T, Causin F, Della Puppa A, Cester G, Mazzai L, Zotti A (2019) Accuracy of deep learning to differentiate the histopathological grading of meningiomas on MR images: a preliminary study. J Magn Reson Imaging 50:1152–1159
Maurer AJ, Safavi-Abbasi S, Cheema AA, Glenn CA, Sughrue ME (2014) Management of petroclival meningiomas: a review of the development of current therapy. J Neurol Surg B Skull Base 75:358–367
Jadid KD, Feychting M, Höijer J, Hylin S, Kihlström L, Mathiesen T (2015) Long-term follow-up of incidentally discovered meningiomas. Acta Neurochir 157:225–230 discussion 230
Paldor I, Awad M, Sufaro YZ, Kaye AH, Shoshan Y (2016) Review of controversies in management of non-benign meningioma. J Clin Neurosci 31:37–46
Mohammad MH, Chavredakis E, Zakaria R, Brodbelt A, Jenkinson MD (2017) A national survey of the management of patients with incidental meningioma in the United Kingdom. Br J Neurosurg 31:459–463
Qiao XJ, Kim HG, Wang DJJ et al (2017) Application of arterial spin labeling perfusion MRI to differentiate benign from malignant intracranial meningiomas. Eur J Radiol 97:31–36
Abdel Razek AAK, Talaat M, El-Serougy L, Gaballa G, Abdelsalam M (2019) Clinical Applications of Arterial Spin Labeling in Brain Tumors. J Comput Assist Tomogr 43:525–532
Acknowledgements
The author(s) would like to thank Ms. Qian Li for the computing resource she provided.
Funding
This study has received funding from the National Natural Science Foundation of China (U21A6005, 81871349), Key-Area Research and Development Program of Guangdong Province (2018B030340001, 2018B030333001), the National Key Research and Development Program of China (2019YFC0118702), the Technology Research and Development Program of Guangdong (2017B090912006), and the Science and Technology Planning Project of Guangzhou City, China (201907010043).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yanqiu Feng.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Ke C, Chen H, Lv X et al (2020) Differentiation between benign and nonbenign meningiomas by using texture analysis from multiparametric MRI. J Magn Reson Imaging 51:1810-1820
Methodology
• retrospective
• experimental
• multicentre study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1.33 MB)
Rights and permissions
About this article
Cite this article
Chen, H., Li, S., Zhang, Y. et al. Deep learning–based automatic segmentation of meningioma from multiparametric MRI for preoperative meningioma differentiation using radiomic features: a multicentre study. Eur Radiol 32, 7248–7259 (2022). https://doi.org/10.1007/s00330-022-08749-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-08749-9