Abstract
Objective
To investigative the performance of intratumoral and peritumoral radiomics based on contrast-enhanced spectral mammography (CESM) to preoperatively predict the effect of the neoadjuvant chemotherapy (NAC) of breast cancers.
Materials and methods
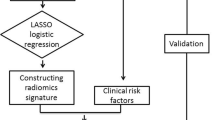
A total of 118 patients with breast cancer who underwent preoperative CESM and NAC from July 2017 to June 2020 were retrospectively analyzed, and the patients were grouped into training (n = 81) and test sets (n = 37) according to the CESM examination time. NAC effect for each patient was assessed by pathology. Intratumoral and peritumoral radiomics features were extracted from CESM images, and feature selection was performed through the Mann–Whitney U test and least absolute shrinkage and selection operator regression (LASSO). Five radiomics signatures based on intratumoral regions, 5-mm peritumoral regions, 10-mm peritumoral regions, intratumoral regions + 5-mm peritumoral regions, and intratumoral regions + 10-mm peritumoral regions were calculated through a linear combination of selected features weighted by their respective coefficients. The prediction performance of radiomics signatures was assessed by the area under the receiver operator characteristic (ROC) curve, the precision-recall (P-R) curve, the calibration curve, and decision curve analysis (DCA).
Results
Ten radiomics features were selected to establish the radiomics signature of intratumoral regions + 5-mm peritumoral regions, which yielded a maximum AUC of 0.85 (95% CI, 0.72–0.98) in the test set. The calibration curves, P-R curves, and DCA showed favorable predictive performance of the five radiomics signatures.
Conclusion
The intratumoral and peritumoral radiomics based on CESM exhibited potential for predicting the NAC effect in breast cancer, which could guide treatment decisions.
Key Points
• The intratumoral and peritumoral CESM-based radiomics signatures show good performance in predicting the NAC effect in breast cancer.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CC:
-
Cranial caudal
- CESM:
-
Contrast-enhanced spectral mammography
- DCA:
-
Decision curve analysis
- ER:
-
Estrogen receptor
- FFDM:
-
Full-field digital mammogram
- HER2:
-
Human epidermal growth factor receptor 2
- ICC:
-
Intraclass correlation coefficient
- LASSO:
-
Least absolute shrinkage and selection operator
- MLO:
-
Mediolateral oblique
- NAC:
-
Neoadjuvant chemotherapy
- PR:
-
Progesterone receptor
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Harbeck N, Gnant M (2017) Breast cancer. Lancet 389(10074):1134–1150
Braman NM, Etesami M, Prasanna P et al (2017) Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res 19(1):57
Bian T, Wu Z, Lin Q et al (2020) Radiomic signatures derived from multiparametric MRI for the pretreatment prediction of response to neoadjuvant chemotherapy in breast cancer. Br J Radiol 93(1115):20200287
Chen S, Liu Y, Ouyang Q-W, Huang L, Luo R-C, Shao Z-M (2015) Clinical and pathological response to neoadjuvant chemotherapy based on primary tumor reduction is correlated to survival in hormone receptor-positive but not hormone receptor-negative locally advanced breast cancer. Ann Surg Oncol 22(1):32–39
James JJ, Tennant SL (2018) Contrast-enhanced spectral mammography (CESM). Clin Radiol 73(8):715–723
Phillips J, Miller MM, Mehta TS et al (2017) Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: patient preferences and attitudes. Clin Imaging 42:193–197
Lee-Felker SA, Tekchandani L, Thomas M et al (2017) Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the evaluation of extent of disease. Radiology 285(2):389–400
Lobbes MBI, Lalji U, Houwers J et al (2014) Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 24(7):1668–1676
Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M (2014) Contrast-enhanced spectral mammography: comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 15(6):689–696
Suter MB, Pesapane F, Agazzi GM et al (2020) Diagnostic accuracy of contrast-enhanced spectral mammography for breast lesions: a systematic review and meta-analysis. Breast 53:8–17
Tang S, Xiang C, Yang Q (2020) The diagnostic performance of CESM and CE-MRI in evaluating the pathological response to neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. Br J Radiol 93(1112):20200301
Sardanelli F, Fallenberg EM, Clauser P et al (2017) Mammography: an update of the EUSOBI recommendations on information for women. Insights Imaging 8(1):11–18
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48(4):441–446
Mao N, Wang Q, Liu M et al (2019) Computerized image analysis to differentiate benign and malignant breast tumors on magnetic resonance diffusion weighted image: a preliminary study. J Comput Assist Tomogr 43(1):93–97
Mao N, Yin P, Li Q et al (2020) Radiomics nomogram of contrast-enhanced spectral mammography for prediction of axillary lymph node metastasis in breast cancer: a multicenter study. Eur Radiol 30(12):6732–6739
Mao N, Yin P, Wang Q et al (2019) Added value of radiomics on mammography for breast cancer diagnosis: a feasibility study. J Am Coll Radiol 16(4 Pt A):485–491
Braman N, Prasanna P, Whitney J et al (2019) Association of peritumoral radiomics with tumor biology and pathologic response to preoperative targeted therapy for HER2 (ERBB2)-positive breast cancer. JAMA Netw Open 2(4):e192561
Liu Z, Li Z, Qu J et al (2019) Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res 25(12):3538–3547
Mao N, Dai Y, Lin F et al (2020) Radiomics nomogram of DCE-MRI for the prediction of axillary lymph node metastasis in breast cancer. Front Oncol 10:541849
Romeo V, Cuocolo R, Apolito R et al (2021) Clinical value of radiomics and machine learning in breast ultrasound: a multicenter study for differential diagnosis of benign and malignant lesions. Eur Radiol 31(12):9511–9519
Pötsch N, Dietzel M, Kapetas P et al (2021) An A.I. classifier derived from 4D radiomics of dynamic contrast-enhanced breast MRI data: potential to avoid unnecessary breast biopsies. Eur Radiol 31(8):5866–5876
Zhang X, Yang Z, Cu W et al (2021) Preoperative prediction of axillary sentinel lymph node burden with multiparametric MRI-based radiomics nomogram in early-stage breast cancer. Eur Radiol 31(8):5924–5939
Wang Z, Lin F, Ma H et al (2021) Contrast-enhanced spectral mammography-based radiomics nomogram for the prediction of neoadjuvant chemotherapy-insensitive breast cancers. Front Oncol 11:605230
Goetz MP, Gradishar WJ, Anderson BO et al (2019) NCCN Guidelines Insights: breast cancer, Version 3.2018. Journal of the National Comprehensive Cancer Network: JNCCN, 17(2), 118–126
GN Hortobagyi, Connolly JL, D’Orsi CJ (2017) AJCC cancer staging manual, Eighth edn. Breast. vol 2018. Springer, New York
Brierley JD, Gospodarowicz MK, Wittekind C (eds.) (2017). TNM classification of malignant tumours. John Wiley & Sons
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163
Hepp T, Schmid M, Gefeller O, Waldmann E, Mayr A (2016) Approaches to regularized regression - a comparison between gradient boosting and the lasso. Methods Inf Med 55(5):422–430
Saito T, Rehmsmeier M (2015) The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One 10(3):e0118432–e0118432
Zhou QM, Zhe L, Brooke RJ, Hudson MM, Yuan Y (2021) A relationship between the incremental values of area under the ROC curve and of area under the precision-recall curve. Diagnostic Prognostic Res 5(1):13–13
Lin F, Wang Z, Zhang K et al (2020) Contrast-enhanced spectral mammography-based radiomics nomogram for identifying benign and malignant breast lesions of sub-1 cm. Front Oncol 10:573630
Comes MC, La Forgia D, Didonna V et al (2021) Early prediction of breast cancer recurrence for patients treated with neoadjuvant chemotherapy: a transfer learning approach on DCE-MRIs. Cancers (Basel) 13(10)
Katayama A, Miligy IM, Shiino S et al (2021) Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2-positive invasive breast cancer. Mod Pathol 34(7):1271–1281
Cortina CS, Gottschalk N, Kulkarni SA, Karst I (2021) Is breast magnetic resonance imaging an accurate predictor of nodal status after neoadjuvant chemotherapy? J Surg Res 257:412–418
Murphy LC, Quinn EM, Razzaq Z et al (2020) Assessing the accuracy of conventional gadolinium-enhanced breast MRI in measuring the nodal response to neoadjuvant chemotherapy (NAC) in breast cancer. Breast J 26(11):2151–2156
Jochelson MS, Dershaw DD, Sung JS et al (2013) Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 266(3):743–751
Cheung Y-C, Lin Y-C, Wan Y-L et al (2014) Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis. Eur Radiol 24(10):2394–2403
La Forgia D, Fanizzi A, Campobasso F et al (2020) Radiomic analysis in contrast-enhanced spectral mammography for predicting breast cancer histological outcome. Diagnostics (Basel) 10(9):708
Losurdo L, Fanizzi A, Basile TMA et al (2019) Radiomics analysis on contrast-enhanced spectral mammography images for breast cancer diagnosis: a pilot study. Entropy 21(11):1110
Vickers AJ, Elkin EB (2006) Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 26(6):565–574
El Adoui M, Drisis S, Benjelloun M (2020) Multi-input deep learning architecture for predicting breast tumor response to chemotherapy using quantitative MR images. Int J Comput Assist Radiol Surg 15(9):1491–1500
Lo Gullo R, Eskreis-Winkler S, Morris EA, Pinker K (2020) Machine learning with multiparametric magnetic resonance imaging of the breast for early prediction of response to neoadjuvant chemotherapy. Breast 49:115–122
Qu Y-H, Zhu H-T, Cao K, Li X-T, Ye M, Sun Y-S (2020) Prediction of pathological complete response to neoadjuvant chemotherapy in breast cancer using a deep learning (DL) method. Thorac Cancer 11(3):651–658
Acknowledgements
Thanks to all participants in this study. Especially thanks to Shaofeng Duan, who is an employee of GE Healthcare, and Ran Zhang, who is an employee of Huiying Medical Technology Co. Ltd, for their contribution in guiding the drafting and revision of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82001775, 61773244), and “Taishan Scholar” Project (NO. tsqn202103197).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Yi Dai.
Conflict of interest
One of the authors of this manuscript (Qianqian Chen) is an employee of GE Healthcare. The remaining authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Qianqian Chen and Haicheng Zhang have significant statistical expertise.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ning Mao and Yinghong Shi are co-first authors on the paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, N., Shi, Y., Lian, C. et al. Intratumoral and peritumoral radiomics for preoperative prediction of neoadjuvant chemotherapy effect in breast cancer based on contrast-enhanced spectral mammography. Eur Radiol 32, 3207–3219 (2022). https://doi.org/10.1007/s00330-021-08414-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08414-7