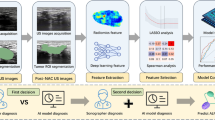
Abstract
Objectives
To develop and validate a multimodality MRI-based radiomics approach to predicting the posttreatment response of lung cancer brain metastases (LCBM) to gamma knife radiosurgery (GKRS).
Methods
We retrospectively analyzed 213 lesions from 137 patients with LCBM who received GKRS between January 2017 and November 2020. The data were divided into a primary cohort (102 patients with 173 lesions) and an independent validation cohort (35 patients with 40 lesions) according to the time of treatment. Benefit result was defined using pretreatment and 3-month follow-up MRI images based on the Response Assessment in Neuro-Oncology Brain Metastases criteria. Valuable radiomics features were extracted from pretreatment multimodality MRI images using random forests. Prediction performance among the radiomics features of tumor core (RFTC) and radiomics features of peritumoral edema (RFPE) together was evaluated separately. Then, the random forest radiomics score and nomogram were developed through the primary cohort and evaluated through an independent validation cohort. Prediction performance was evaluated by ROC curve, calibration curve, and decision curve.
Results
Gender (p = 0.018), histological subtype (p = 0.009), epidermal growth factor receptor mutation (p = 0.034), and targeted drug treatment (p = 0.021) were significantly associated with posttreatment response. Adding RFPE to RFTC showed improved prediction performance than RFTC alone in primary cohort (AUC = 0.848 versus AUC = 0.750; p < 0.001). Finally, the radiomics nomogram had an AUC of 0.930, a C-index of 0.930 (specificity of 83.1%, sensitivity of 87.3%) in primary cohort, and an AUC of 0.852, a C-index of 0.848 (specificity of 84.2%, sensitivity of 76.2%) in validation cohort.
Conclusions
Multimodality MRI-based radiomics models can predict the posttreatment response of LCBM to GKRS.
Key Points
• Among the selected radiomics features, texture features basically contributed the dominant force in prediction tasks (80%), especially gray-level co-occurrence matrix features (40%).
• Adding RFPE to RFTC showed improved prediction performance than RFTC alone in primary cohort (AUC = 0.848 versus AUC = 0.750; p < 0.001).
• The multimodality MRI-based radiomics nomogram showed high accuracy for distinguishing the posttreatment response of LCBM to GKRS (AUC = 0.930, in primary cohort; AUC = 0.852, in validation cohort).





Similar content being viewed by others
Abbreviations
- CBV:
-
Cerebral blood volume
- EGFR:
-
Epidermal growth factor receptor
- GKRS:
-
Gamma knife radiosurgery
- LCBM:
-
Lung cancer brain metastases
- RF_Score:
-
Random forest radiomics score
- RFPE:
-
Radiomics features of peritumoral edema
- RFTC:
-
Radiomics features of tumor core
- T1-MPRAGE:
-
T1 magnetization-prepared rapid gradient-echo
References
Bowden G, Kano H, Caparosa E et al (2015) Gamma knife radiosurgery for the management of cerebral metastases from non-small cell lung cancer. J Neurosurg 122:766–772. https://doi.org/10.3171/2014.12.JNS141111
Taunk NK, Oh JH, Shukla-Dave A et al (2018) Early posttreatment assessment of MRI perfusion biomarkers can predict long-term response of lung cancer brain metastases to stereotactic radiosurgery. Neuro Oncol 20:567–575. https://doi.org/10.1093/neuonc/nox159
Taunk NK, Oh JH, Dave A et al (2017) Early posttreatment assessment of MRI perfusion biomarkers can predict long-term response of NSCLC brain metastases to SRS: a longitudinal analysis. Int J Radiat Oncol Biol Phys 99:S84. https://doi.org/10.1016/j.ijrobp.2017.06.203
Fehr D, Veeraraghavan H, Wibmer A et al (2015) Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc Natl Acad Sci U S A 112:E6265–E6273. https://doi.org/10.1073/pnas.1505935112
Nie K, Shi L, Chen Q et al (2016) Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI. Clin Cancer Res 22:5256–5264. https://doi.org/10.1158/1078-0432.CCR-15-2997
Yang WC, Xiao F, Shih JY et al (2018) Epidermal growth factor receptor mutation predicts favorable outcomes in non-small cell lung cancer patients with brain metastases treated with stereotactic radiosurgery. Radiother Oncol 126:368–374. https://doi.org/10.1016/j.radonc.2017.10.010
Zindler JD, Jochems A, Lagerwaard FJ et al (2017) Individualized early death and long-term survival prediction after stereotactic radiosurgery for brain metastases of non-small cell lung cancer: two externally validated nomograms. Radiother Oncol 123:189–194. https://doi.org/10.1016/j.radonc.2017.02.006
Moraes FY, Winter J, Atenafu EG et al (2019) Outcomes following stereotactic radiosurgery for small to medium-sized brain metastases are exceptionally dependent upon tumor size and prescribed dose. Neuro Oncol 21:242–251. https://doi.org/10.1093/neuonc/noy159
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. https://doi.org/10.1148/radiol.2015151169
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762. https://doi.org/10.1038/nrclinonc.2017.141
Bi WL, Hosny A, Schabath MB et al (2019) Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. https://doi.org/10.3322/caac.21552
Nasief H, Zheng C, Schott D et al (2019) A machine learning based delta-radiomics process for early prediction of treatment response of pancreatic cancer. NPJ Precis Oncol 3:1–10. https://doi.org/10.1038/s41698-019-0096-z
Beukinga RJ, Hulshoff JB, Mul VEM et al (2018) Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging 18F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology 287:983–992. https://doi.org/10.1148/radiol.2018172229
Horvat N, Veeraraghavan H, Khan M et al (2018) MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 287:833–843. https://doi.org/10.1148/radiol.2018172300
Kickingereder P, Götz M, Muschelli J et al (2016) Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res 22:5765–5771. https://doi.org/10.1158/1078-0432.CCR-16-0702
Carroll TJ, Horowitz S, Shin W et al (2008) Quantification of cerebral perfusion using the “bookend technique”: an evaluation in CNS tumors. Magn Reson Imaging 26(10):1352–1359. https://doi.org/10.1016/j.mri.2008.04.010
Lin NU, Lee EQ, Aoyama H et al (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Tustison NJ, Avants BB, Cook PA et al (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320. https://doi.org/10.1109/TMI.2010.2046908
Yushkevich PA, Piven J, Hazlett HC et al (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Strobl C, Boulesteix AL, Zeileis A, Hothorn T (2007) Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics 8: https://doi.org/10.1186/1471-2105-8-25
Schapire RE (2001) Random forests. Mach Learn 45:5–32. https://doi.org/10.1023/A:1010933404324
Janitza S, Hornung R (2018) On the overestimation of random forest’s out-of-bag error. PLoS One 13: https://doi.org/10.1371/journal.pone.0201904
Touw WG, Bayjanov JR, Overmars L et al (2013) Data mining in the life science swith random forest: a walk in the park or lost in the jungle? Brief Bioinform 14:315–326. https://doi.org/10.1093/bib/bbs034
Chalkidou A, O’Doherty MJ, Marsden PK (2015) False discovery rates in PET and CT studies with texture features: a systematic review. PLoS One 10: https://doi.org/10.1371/journal.pone.0124165
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837. https://doi.org/10.2307/2531595
Demler OV, Pencina MJ, D’Agostino RB (2012) Misuse of DeLong test to compare AUCs for nested models. Stat Med 31:2577–2587. https://doi.org/10.1002/sim.532827
Pedregosa F, Varoquaux G, Gramfort A et al (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830
Hatt M, Majdoub M, Vallières M et al (2015) 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 56:38–44. https://doi.org/10.2967/jnumed.114.144055
Park JE, Kim D, Kim HS et al (2020) Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol 30:523–536. https://doi.org/10.1007/s00330-019-06360-z
Park JE, Kim HS, Kim D et al (2020) A systematic review reporting quality of radiomics research in neuro-oncology: toward clinical utility and quality improvement using high-dimensional imaging features. BMC Cancer 20(1):1–11. https://doi.org/10.1186/s12885-019-6504-5
Prasanna P, Patel J, Partovi S, Madabhushi A, Tiwari (2017) Erratum to: Radiomic features from the peritumoral brain parenchyma on treatmentnaïve multiparametric MR imaging predict long versus shortterm survival in glioblastoma multiforme: preliminary findings. Eur Radiol 27:4198–4199. https://doi.org/10.1007/s00330-017-4815-y
Rathore S, Akbari H, Doshi J et al (2018) Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma: implications for personalized radiotherapy planning. J Med Imaging (Bellingham) 5:1. https://doi.org/10.1117/1.jmi.5.2.021219
Arvanitis CD, Ferraro GB, Jain RK (2020) The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 20:26–41. https://doi.org/10.1038/s41568-019-0205-x
Win T, Miles KA, Janes SM et al (2013) Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res 19:3591–3599. https://doi.org/10.1158/1078-0432.CCR-12-1307
Zamboglou C, Carles M, Fechter T et al (2019) Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer – a comparison study with histology reference. Theranostics 9:2595–2605. https://doi.org/10.7150/thno.32376
Mohlin S, Wigerup C, Jögi A, Påhlman S (2017) Hypoxia, pseudohypoxia and cellular differentiation. Exp Cell Res 356:192–196. https://doi.org/10.1016/j.yexcr.2017.03.007
Nandu H, Wen PY, Huang RY (2018) Imaging in neuro-oncology. Ther Adv Neurol Disord 11:1–19. https://doi.org/10.1177/1756286418759865
Park JE, Kim D, Kim HS et al (2020) Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol 30(1):523–536. https://doi.org/10.1007/s00330-019-06360-z
Funding
This study has received funding by the National Natural Science Foundation of China (No. 61971271), the Natural Science Foundation of Shandong Province (No. ZR2019QF007), Primary Research and Development Plan of Shandong Province (2017CXCG1209, 2018GGX101018, 2019QYTPY020), and the Taishan Scholars Program (No. tsqn 20161023, 20161070).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yingchao Liu.
Conflict of interest
The authors declare no competing interests.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained (NSFC NO: 2019–272).
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Z., Wang, B., Han, X. et al. Multimodality MRI-based radiomics approach to predict the posttreatment response of lung cancer brain metastases to gamma knife radiosurgery. Eur Radiol 32, 2266–2276 (2022). https://doi.org/10.1007/s00330-021-08368-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08368-w