Abstract
Objectives
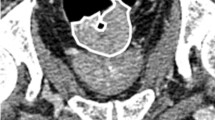
There are individual variations in neo-adjuvant chemoradiation therapy (nCRT) in patients with locally advanced rectal cancer (LARC). No reliable modality currently exists that can predict the efficacy of nCRT. The purpose of this study is to assess if CT-based fractal dimension and filtration-histogram texture analysis can predict therapeutic response to nCRT in patients with LARC.
Methods
In this retrospective study, 215 patients (average age: 57 years (18–87 years)) who received nCRT for LARC between June 2005 and December 2016 and underwent a staging diagnostic portal venous phase CT were identified. The patients were randomly divided into two datasets: a training set (n = 170), and a validation set (n = 45). Tumor heterogeneity was assessed on the CT images using fractal dimension (FD) and filtration-histogram texture analysis. In the training set, the patients with pCR and non-pCR were compared in univariate analysis. Logistic regression analysis was applied to identify the predictive value of efficacy of nCRT and receiver operating characteristic analysis determined optimal cutoff value. Subsequently, the most significant parameter was assessed in the validation set.
Results
Out of the 215 patients evaluated, pCR was reached in 20.9% (n = 45/215) patients. In the training set, 7 out of 37 texture parameters showed significant difference comparing between the pCR and non-pCR groups and logistic multivariable regression analysis incorporating clinical and 7 texture parameters showed that only FD was associated with pCR (p = 0.001). The area under the curve of FD was 0.76. In the validation set, we applied FD for predicting pCR and sensitivity, specificity, and accuracy were 60%, 89%, and 82%, respectively.
Conclusion
FD on pretreatment CT is a promising parameter for predicting pCR to nCRT in patients with LARC and could be used to help make treatment decisions.
Key Points
• Fractal dimension analysis on pretreatment CT was associated with response to neo-adjuvant chemoradiation in patients with locally advanced rectal cancer.
• Fractal dimension is a promising biomarker for predicting pCR to nCRT and may potentially select patients for individualized therapy.





Similar content being viewed by others
Abbreviations
- FD:
-
Fractal dimension
- LARC:
-
Locally advanced rectal cancer
- MPP:
-
Mean positive pixel
- nCRT:
-
Neo-adjuvant chemoradiation
- pCR:
-
Pathological complete response
- SD:
-
Standard deviation
- SSF:
-
Spatial scaling filter
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019 (US statistics). CA Cancer J Clin 69(1):7–34
Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33
Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U (2005) Swedish rectal cancer trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 23(24):5644–5650
Schmoll HJ, Van Cutsem E, Stein A et al (2012) Esmo consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 23(10):2479–2516
Sauer R, Liersch T, Merkel S et al (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30(16):1926–1933
Maas M, Nelemans P, Valentini V et al (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11(9):835–844
Renehan AG, Malcomson L, Emsley R et al (2016) Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 17(2):174–183
Davnall F, Yip CSP, Ljungqvist G et al (2012) Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3(6):573–589
Gerlinger M (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 65(4):883–892
Turner NC, Reis-Filho JS (2012) Genetic heterogeneity and cancer drug resistance. Lancet Oncol 13(4):e178–e185
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13(3):400–406
Hayano K, Tian F, Kambadakone AR et al (2015) Texture analysis of non-contrast-enhanced computed tomography for assessing angiogenesis and survival of soft tissue sarcoma”. J Comput Assist Tomogr 39(4):607–612
Noda Y, Goshima S, Tsuji Y et al (2019) Prognostic evaluation of pancreatic ductal adenocarcinoma: associations between molecular biomarkers and CT imaging findings. Pancreatology 19(2):331–339
Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K (2012) Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol 67(2):157–164
Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K (2012) Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol 22(4):796–802
Yin JD, Song LR, Lu HC, Zheng X (2020) Prediction of different stages of rectal cancer: texture analysis based on diffusion-weighted images and apparent diffusion coefficient maps. World J Gastroenterol 26(17):2082–2096
Nardone V, Reginelli Alfonso, Scala Fernando et al (2019) Magnetic-resonance-imaging texture analysis predicts early progression in rectal cancer patients undergoing neoadjuvant chemoradiation. Gastroenterol Res Pract. https://doi.org/10.1155/2019/8505798
Shu Z, Fang Songhua, Ye Qin et al (2019) Prediction of efficacy of neoadjuvant chemoradiotherapy for rectal cancer: the value of texture analysis of magnetic resonance images. Abdom Radiol (NY). https://doi.org/10.1007/s00261-019-01971-y
Chee CG, Kim YH, Lee KH et al (2017) CT texture analysis in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: a potential imaging biomarker for treatment response and prognosis. PLoS One 12(8):1–12
Vandendorpe B, Durot C, Lebellec L et al (2019) Prognostic value of the texture analysis parameters of the initial computed tomographic scan for response to neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer. Radiother Oncol 135:153–160
Mandelbrot BB (1982) Fractal geometry. W.H.Freeman and Company New York
Kurata Y, Hayano K, Ohira G, Narushima K, Aoyagi T, Matsubara H (2018) Fractal analysis of contrast-enhanced CT images for preoperative prediction of malignant potential of gastrointestinal stromal tumor. Abdom Radiol (NY) 43(10):2659–2664
Hayano K, Lee SH, Yoshida H, Zhu AX, Sahani DV (2014) Fractal analysis of CT perfusion images for evaluation of antiangiogenic treatment and survival in hepatocellular carcinoma. Acad Radiol 21(5):654–660
Hayano K, Yoshida H, Zhu AX, Sahani DV (2014) Fractal analysis of contrast-enhanced CT images to predict survival of patients with hepatocellular carcinoma treated with sunitinib. Dig Dis Sci 59(8):1996–2003
Abramyuk A, Wolf G, Shakirin G et al (2010) Preliminary assessment of dynamic contrast-enhanced CT implementation in pretreatment FDG-PET/CT for outcome prediction in head and neck tumors. Acta Radiol 51(7):793–799
Ganeshan B, Miles KA (2013) Quantifying tumour heterogeneity with CT. Cancer Imaging 13(1):140–149
Nelson DA, White E, Tan T-T, Rabson AB, Anderson D, Degenhardt K (2004) Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev 18(17):2095–2107
Russnes HG, Hicks J, Borresen-Dale A-L, Navin N (2011) Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Inves 121(10):3810
Teicher BA (1995) Physiologic mechanisms of therapeutic resistance. Blood flow and hypoxia. Hematol Oncol Clin North Am 9(2):475–506
Sloothaak DA, Geijsen DE, van Leersum NJ et al (2013) Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 100(7):933–939
Grumann MM, Noack EM, Hoffmann IA, Schlag PM (2001) Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg 233(2):149–156
Kamran SC, Lennerz J, Margolis C et al (2019) Integrative molecular characterization of resistance to neoadjuvant chemoradiation in rectal cancer. Clin Cancer Res 25(18):5561–5571
García-Figueiras R, Baleato-González S, Padhani AR et al (2018) Advanced imaging techniques in evaluation of colorectal cancer. Radiographics 38(3):740–765
Miles KA, Ganeshan B, Griffiths MR, Young RCD, Chatwin CR (2009) Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 250(2):444–452
Win T, Miles KA, Janes SM et al (2013) Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res 19(13):3591–3599
Dohan A, Gallix B, Guiu B et al (2020) Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut 69(3):531–539
Yasaka K, Akai Hiroyuki, Mackin Dennis et al (2017) Precision of quantitative computed tomography texture analysis using image filtering: a phantom study for scanner variability. Medicine (Baltimore) 96(21):e6993
Chen S, Ganeshan B, Fraioli F (2016) Reproducibility of CT Texture parameters by leveraging publicly available patient imaging datasets. In Radiological Society of North America, Chicago
Ng F, Kozarski R, Ganeshan B, Goh V (2013) Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 82(2):342–348
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Avinash Kambadakone.
Conflict of interest
Avinash Kambadakone: Research Grants (GE Healthcare, Philips Healthcare and PanCAN), Advisory Board (Bayer).
All other authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tochigi, T., Kamran, S.C., Parakh, A. et al. Response prediction of neoadjuvant chemoradiation therapy in locally advanced rectal cancer using CT-based fractal dimension analysis. Eur Radiol 32, 2426–2436 (2022). https://doi.org/10.1007/s00330-021-08303-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08303-z