Abstract
Objectives
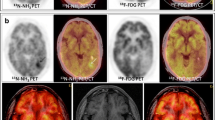
The aim of this work was investigating the methods based on coupling cerebral perfusion (ASL) and amino acid metabolism ([18F]DOPA-PET) measurements to evaluate the diagnostic performance of PET/MRI in glioma follow-up.
Methods
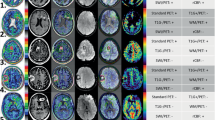
Images were acquired using a 3-T PET/MR system, on a prospective cohort of patients addressed for possible glioma progression. Data were preprocessed with statistical parametric mapping (SPM), including registration on T1-weighted images, spatial and intensity normalization, and tumor segmentation. As index tests, tumor isocontour maps of [18F]DOPA-PET and ASL T-maps were created and metabolic/perfusion abnormalities were evaluated with the asymmetry index z-score. SPM map analysis of significant size clusters and semi-quantitative PET and ASL map evaluation were performed and compared to the gold standard diagnosis. Lastly, ASL and PET topography of significant clusters was compared to that of the initial tumor.
Results
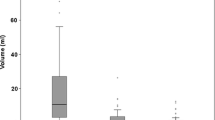
Fifty-eight patients with unilateral treated glioma were included (34 progressions and 24 pseudo-progressions). The tumor isocontour maps and T-maps showed the highest specificity (100%) and sensitivity (94.1%) for ASL and [18F]DOPA analysis, respectively. The sensitivity of qualitative SPM maps and semi-quantitative rCBF and rSUV analyses were the highest for glioblastoma.
Conclusion
Tumor isocontour T-maps and combined analysis of CBF and [18F]DOPA-PET uptake allow achieving high diagnostic performance in differentiating between progression and pseudo-progression in treated gliomas. The sensitivity is particularly high for glioblastomas.
Key Points
• Applied separately, MRI and PET imaging modalities may be insufficient to characterize the brain glioma post-therapeutic profile.
• Combined ASL and [18F]DOPA-PET map analysis allows differentiating between tumor progression and pseudo-progression.






Similar content being viewed by others
Abbreviations
- [18F]DOPA:
-
18F-Fluoro-3.4-dihydroxy-l-phenylalanine
- [18F]FDG:
-
18F-Fluoro-deoxy-glucose
- ASL:
-
Arterial spin labeling
- CBF:
-
Cerebral blood flow
- CBV:
-
Cerebral blood volume
- DSC:
-
Dynamic susceptibility contrast
- FLAIR:
-
Fluid-attenuated inversion recovery
- FN:
-
False negative
- FP:
-
False positive
- MRI:
-
Magnetic resonance imaging
- pCASL:
-
Pseudo-continuous spin-echo ASL
- PET:
-
Positron emission tomography
- PWI:
-
Perfusion-weighted imaging
- rCBF:
-
Cerebral blood flow ratio
- rSUV:
-
Standardized uptake value ratio
- SE:
-
Spin-echo
- SPM:
-
Statistical parametric mapping
- SUV:
-
Standardized uptake value
- TN:
-
True negative
- TP:
-
True positive
- VOI:
-
Volume of interest
- WHO:
-
World Health Organization
- zAI:
-
Z-score of the asymmetry index
References
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY (2012) Primary brain tumours in adults. Lancet Lond Engl 379:1984–1996
Klobukowski L, Falkov A, Chelimo C, Fogh SE (2018) A retrospective review of re-irradiating patients’ recurrent high-grade gliomas. Clin Oncol (R Coll Radiol). https://doi.org/10.1016/j.clon.2018.05.004
Thust SC, Heiland S, Falini A et al (2018) Glioma imaging in Europe: a survey of 220 centres and recommendations for best clinical practice. Eur Radiol 28:3306–3317
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Mullins ME, Barest GD, Schaefer PW et al (2005) Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol 26:1967–1972
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Mabray MC, Barajas RF, Cha S (2015) Modern brain tumor imaging. Brain Tumor Res Treat 3:8–23
Rossi A, Gandolfo C, Morana G, Severino M, Garrè ML, Cama A (2010) New MR sequences (diffusion, perfusion, spectroscopy) in brain tumours. Pediatr Radiol 40:999–1009
Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X (2016) Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 281:337–356
Galldiks N, Stoffels G, Ruge MI et al (2013) Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J Nucl Med 54:2046–2054
Pirotte BJM, Levivier M, Goldman S et al (2009) Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 64:471–481 discussion 481
Brandes AA, Tosoni A, Spagnolli F et al (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 10:361–367
Boscolo Galazzo I, Mattoli MV, Pizzini FB et al (2016) Cerebral metabolism and perfusion in MR-negative individuals with refractory focal epilepsy assessed by simultaneous acquisition of (18)F-FDG PET and arterial spin labeling. Neuroimage Clin 11:648–657
Storti SF, Boscolo Galazzo I, Del Felice A et al (2014) Combining ESI, ASL and PET for quantitative assessment of drug-resistant focal epilepsy. Neuroimage 102(Pt 1):49–59
Morana G, Piccardo A, Tortora D et al (2017) Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F-DOPA PET. Eur J Nucl Med Mol Imaging 44:2084–2093
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116
Brett M, Leff AP, Rorden C, Ashburner J (2001) Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14:486–500
Lim Y-M, Cho Y-W, Shamim S et al (2008) Usefulness of pulsed arterial spin labeling MR imaging in mesial temporal lobe epilepsy. Epilepsy Res 82:183–189
Chen W, Silverman DHS, Delaloye S et al (2006) 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47:904–911
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Komori T (2017) The 2016 WHO classification of tumours of the central nervous system: the major points of revision. Neurol Med Chir (Tokyo) 57:301–311
Bergamino M, Saitta L, Barletta L et al (2013) Measurement of blood-brain barrier permeability with t1-weighted dynamic contrast-enhanced MRI in brain tumors: a comparative study with two different algorithms. ISRN Neurosci 2013:905279
Dolui S, Vidorreta M, Wang Z et al (2017) Comparison of PASL, PCASL, and background-suppressed 3D PCASL in mild cognitive impairment. Hum Brain Mapp 38:5260–5273
Gai ND, Chou YY, Pham D, Butman JA (2017) Reduced distortion artifact whole brain CBF mapping using blip-reversed non-segmented 3D echo planar imaging with pseudo-continuous arterial spin labeling. Magn Reson Imaging 44:119–124
Lindner T, Ahmeti H, Juhasz J et al (2018) A comparison of arterial spin labeling and dynamic susceptibility perfusion imaging for resection control in glioblastoma surgery. Oncotarget 9:18570–18577
Galldiks N, Langen K-J, Holy R et al (2012) Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med 53:1048–1057
Pafundi DH, Laack NN, Youland RS et al (2013) Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol 15:1058–1067
Furtner J, Bender B, Braun C et al (2014) Prognostic value of blood flow measurements using arterial spin labeling in gliomas. PLoS One 9:e99616
Salber D, Stoffels G, Pauleit D et al (2007) Differential uptake of O-(2-18F-fluoroethyl)-L-tyrosine, L-3H-methionine, and 3H-deoxyglucose in brain abscesses. J Nucl Med 48:2056–2062
Langen K-J, Bröer S (2004) Molecular transport mechanisms of radiolabeled amino acids for PET and SPECT. J Nucl Med 45:1435–1436
Gasparetto EL, Pawlak MA, Patel SH et al (2009) Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology 250:887–896
Filss CP, Cicone F, Shah NJ, Galldiks N, Langen K-J (2017) Amino acid PET and MR perfusion imaging in brain tumours. Clin Transl Imaging 5:209–223
Wang Y-F, Hou B, Yang S-J et al (2016) Diagnostic significance of arterial spin labeling in the assessment of tumor grade in brain. J Cancer Res Ther 12:259–266
Kinahan PE, Fletcher JW (2010) Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR 31:496–505
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Arnaud Pellerin, the corresponding author.
Conflict of interest
The authors of this manuscript declare relationships with the following company:
Maya Khalifé received a research grant from General Electric Healthcare. No other financial relationships that might lead to a perceived conflict of interest relevant to this article were reported.
Statistics and biometry
One of the authors has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study. All data were extracted from the local database of PET/MR studies, which was approved by the French authority for the protection of privacy and personal data in clinical research (CNIL, No. 2111722). This study was performed according to the principles of the Declaration of Helsinki.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study/observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pellerin, A., Khalifé, M., Sanson, M. et al. Simultaneously acquired PET and ASL imaging biomarkers may be helpful in differentiating progression from pseudo-progression in treated gliomas. Eur Radiol 31, 7395–7405 (2021). https://doi.org/10.1007/s00330-021-07732-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07732-0