Abstract
Objectives
This study evaluated the characteristics of left ventricular maximum principal strain (LV-MPS) using cardiac CT in subjects with normal LV function.
Methods
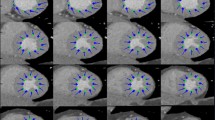
Of 973 subjects who underwent retrospective electrocardiogram-gated cardiac CT using a third-generation dual-source CT without beta-blocker administration, 31 subjects with preserved LV ejection fraction ≥ 55% assessed by echocardiography without coronary artery stenosis and cardiac pathology were retrospectively identified. CT images were reconstructed every 5% (0–95%) of the RR interval. LV-MPS and the time to peak (TTP) were analyzed using the 16-segment model and compared among three levels (base, mid, and apex) and among four regions (anterior, septum, inferior, and lateral) using the Steel–Dwass test. The intra- and inter-observer reproducibilities for LV-MPS were calculated using intraclass correlation coefficients (ICCs).
Results
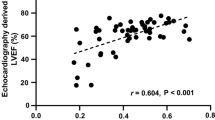
The intra- and inter-observer ICCs (95% confidence interval) for peak LV-MPS were 0.96 (0.94–0.97) and 0.94 (0.92–0.96), respectively. The global peak LV-MPS (median, inter-quantile range) was 0.59 (0.55–0.72). The regional LV-MPS significantly increased in the order of the basal (0.54, 0.49–0.59), mid-LV (0.57, 0.53–0.65), and apex (0.68, 0.60–0.84) (p < 0.05, in each), and was significantly higher in the lateral wall (0.66, 0.60–0.77), while that in the septal region (0.47, 0.44–0.54) was the lowest among the four LV regions (all p < 0.05). No significant difference in TTP was seen among the myocardial levels and regions.
Conclusion
CT-derived LV-MPS is reproducible and quantitatively represents synchronized myocardial contraction with heterogeneous values in subjects with normal LV function.
Key Points
• CT-derived left ventricular maximum principal strain analysis allows highly reproducible quantitative assessments of left ventricular myocardial contraction.
• In subjects with normal cardiac function, the peak value of CT-derived left ventricular maximum principal strain is the highest in the apical level and in the lateral wall and the lowest in the septum.
• The regional peak left ventricular maximum principal strain shows intra-ventricular heterogeneity on a per-patient basis, but myocardial contraction is globally synchronized in subjects with normal cardiac function seen on cardiac CT.





Similar content being viewed by others
Abbreviations
- %SWT:
-
Percent systolic wall thickening
- 2D:
-
Two-dimensional
- EF:
-
Ejection fraction
- GLS:
-
Global longitudinal strain
- LV:
-
Left ventricular
- MPS:
-
Maximum principal strain
- STE:
-
Speckle-tracking echocardiography
- TTP:
-
Time to peak
References
Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S (2016) Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 37:1196–1207
Leitman M, Lysiansky M, Lysyansky P et al (2010) Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr 23:64–70
Jeung MY, Germain P, Croisille P, El Ghannudi S, Roy C, Gangi A (2012) Myocardial tagging with MR imaging: overview of normal and pathologic findings. Radiographics 32:1381–1398
Zhang KW, French B, May Khan A et al (2014) Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc. https://doi.org/10.1161/JAHA.113.000550
Götte MJ, van Rossum AC, Twisk JWR, Kuijer JPA, Marcus JT, Visser CA (2001) Quantification of regional contractile function after infarction: strain analysis superior to wall thickening analysis in discriminating infarct from remote myocardium. J Am Coll Cardiol 37:808–817
Miller JM, Rochitte CE, Dewey M et al (2008) Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 359:2324–2336
Rochitte CE, George RT, Chen MY et al (2014) Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J 35:1120–1130
Tavakoli V, Sahba N (2014) Cardiac motion and strain detection using 4D CT images: comparison with tagged MRI, and echocardiography. Int J Cardiovasc Imaging 30:175–184
Tee MW, Won S, Raman FS et al (2015) Regional strain analysis with multidetector CT in a swine cardiomyopathy model: relationship to cardiac MR tagging and myocardial fibrosis. Radiology 277:88–94
Buss SJ, Schulz F, Mereles D et al (2014) Quantitative analysis of left ventricular strain using cardiac computed tomography. Eur J Radiol 83:e123–e130
Fukui M, Xu J, Abdelkarim I et al (2019) Global longitudinal strain assessment by computed tomography in severe aortic stenosis patients - feasibility using feature tracking analysis. J Cardiovasc Comput Tomogr 13:157–162
Croisille P, Moore CC, Judd RM et al (1999) Differentiation of viable and nonviable myocardium by the use of three-dimensional tagged MRI in 2-day-old reperfused canine infarcts. Circulation 99:284–291
Pilla JJ, Koomalsingh KJ, McGarvey JR et al (2015) Regional myocardial three-dimensional principal strains during postinfarction remodeling. Ann Thorac Surg 99:770–778
Tanabe Y, Kido T, Kurata A et al (2017) Three-dimensional maximum principal strain using cardiac computed tomography for identification of myocardial infarction. Eur Radiol 27:1667–1675
Marwan M, Ammon F, Bittner D et al (2018) CT-derived left ventricular global strain in aortic valve stenosis patients: a comparative analysis pre and post transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 12:240–244
Schmitt B, Li T, Kutty S et al (2015) Effects of incremental beta-blocker dosing on myocardial mechanics of the human left ventricle: MRI 3D-tagging insight into pharmacodynamics supports theory of inner antagonism. Am J Physiol Heart Circ Physiol 309:H45–H52
Motoki H, Koyama J, Izawa A et al (2014) Impact of azelnidipine and amlodipine on left ventricular mass and longitudinal function in hypertensive patients with left ventricular hypertrophy. Echocardiography 31:1230–1238
Harrington JK, Richmond ME, Fein AW, Kobsa S, Satwani P, Shah A (2018) Two-dimensional speckle tracking echocardiography-derived strain measurements in survivors of childhood cancer on angiotensin converting enzyme inhibition or receptor blockade. Pediatr Cardiol 39:1404–1412
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 26:185–191
Lang RM, Bierig M, Devereux RB et al (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Kawaguchi N, Kurata A, Kido T et al (2014) Optimization of coronary attenuation in coronary computed tomography angiography using diluted contrast material. Circ J 78:662–670
Ammon F, Bittner D, Hell M et al (2019) CT-derived left ventricular global strain: a head-to-head comparison with speckle tracking echocardiography. Int J Cardiovasc Imaging 35:1701–1707
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Del-Canto I, López-Lereu MP, Monmeneu JV et al (2015) Characterization of normal regional myocardial function by MRI cardiac tagging. J Magn Reson Imaging 41:83–92
McVeigh ER, Pourmorteza A, Guttman M et al (2018) Regional myocardial strain measurements from 4DCT in patients with normal LV function. J Cardiovasc Comput Tomogr 12:372–378
Shiina Y, Inai K, Takahashi T, Shimomiya Y, Nagao M (2020) Clinical impact of cardiac computed tomography derived three-dimensional strain for adult congenital heart disease: a pilot study. Int J Cardiovasc Imaging 36:131–140
LeWinter MM, Kent RS, Kroener JM, Carew TE, Covell JW (1975) Regional differences in myocardial performance in the left ventricle of the dog. Circ Res 37:191–199
Liu W, Ashford MW, Chen J (2006) MR tagging demonstrates quantitative differences in regional ventricular wall motion in mice, rats, and men. Am J Physiol Heart Circ Physiol 291:H2515–H2521
Ashford MW Jr, Liu W, Lin SJ et al (2005) Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation 16:2462–2467
Amzulescu MS, De Craene M, Langet H et al (2019) Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 20:605–619
Nahum J, Bensaid A, Dussault C et al (2010) Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging 3:249–256
Park JJ, Park JB, Park JH, Cho GY (2018) Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 71:1947–1957
Augustine D, Lewandowski AJ, Lazdam M et al (2013) Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson. https://doi.org/10.1186/1532-429X-15-8
Kleijn SA, Pandian NG, Thomas JD et al (2015) Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging 16:410–416
Stocker TJ, Deseive S, Leipsic J et al (2018) Reduction in radiation exposure in cardiovascular computed tomography imaging: results from the PROspective multicenter registry on radiaTion dose estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur Heart J 39:3715–3723
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Teruhito Mochizuki.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding information
The authors state that this work has not received any funding.
Statistics and biometry
One of the authors has significant statistical expertise (Dr. Akira Kurata).
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoshida, K., Tanabe, Y., Kido, T. et al. Characteristics of the left ventricular three-dimensional maximum principal strain using cardiac computed tomography: reference values from subjects with normal cardiac function. Eur Radiol 30, 6109–6117 (2020). https://doi.org/10.1007/s00330-020-07001-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07001-6