Abstract
To enhance Mangiferin (MNG) the brain bioavailability by developed novel polysorbate-80 (P 80) MNG PLGA NPs and examined the quantity of MNG through developed a novel method of LC–MS/MS in the ischemic rat brain treatment. The solvent evaporation method used to develop a novel MNG loaded PLGA NPs followed by their coating from polysorbate-80. Polysorbate-80 (P 80) MNG PLGA NPs developed based on EE, particle size, Zeta Potential, PDI, and loading capacity with their characterization followed by drug release and intranasal permeation to enhance the brain bioavailability and also determined their neurobehavioral as well as biochemical evaluation with the histopathological examination. P 80 MNG PLGA NPs were optimized with their particle size 103.4 ± 2.66 nm, PDI: 0.201 ± 0.008, Zeta Potential (−35.8 ± 2.48), and drug loading 37.16 ± 2.09% with 76.08 ± 4.91% entrapment efficiency showed a sustained and controlled release (83.43 ± 6.47%) with great permeation (> 83%) of MNG. MNG & IS showed retention time (0.756 and 1.258 min) and their m/z (421.20/301.20 and 237.2/179.2), respectively. MNG showed a good linearity range, i.e., 10.0–1000.0 ng mL−1; the results of inter-and-intraday accuracy and CV were found to be (93.01–99.43%) and (2.27–4.02%), respectively. An excellent significant results was showed, i.e., p < 0.001 for (AUC)0–24 & Cmax via i.n. dose delivered. A highly significantly results of P 80 MNG PLGA NPs (i.n.) were found based on the examination of biochemical, neurobehavioral, and histopathological in the developed ischemic MCAO brain rat’s model. An excellent significant role of P 80 MNG PLGA NPs for MNG were proved based on enhancement of brain bioavailability of MNG via i.n. delivery of the rats and targeted easily to the brain in the treatment of cerebral ischemia followed by improvement of neuroprotection based on use of a very small dose of MNG.
Graphical Abstract















Similar content being viewed by others
Abbreviations
- MNG:
-
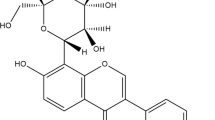
Mangiferin
- P 80:
-
Polysorbate-80
- PLGA:
-
Poly (lactic-co-glycolic acid)
- NPs:
-
Nanoparticles
- LC–MS/MS:
-
Liquid chromatography-mass spectrometry/mass spectrometry
- EE:
-
Entrapment/encapsulation efficiency
- PDI:
-
Polydispersity index
- ZP:
-
Zeta Potential
- CV:
-
Coefficient of variation
- AUC:
-
Area under curve
- i.v.:
-
Intravenous
- i.n.:
-
Intranasal
- MCAO:
-
Middle cerebral artery occlusion
- LOQQC:
-
Lower limit of quantification of quality control
- LQC:
-
Lower-level quality control
- MQC:
-
Middle level quality control
- HQC:
-
Higher level quality control
- RT:
-
Retention time
- ROS:
-
Reactive oxygen species
- DCM:
-
Dichloromethane
- SEM:
-
Scanning electron microscopy
- DSC:
-
Differential scanning calorimetry
- TDDS:
-
Targeted drug delivery system
- PK:
-
Pharmacokinetic
- SMA:
-
Spontaneous motor activity
- FT:
-
Flexion test
- GPx:
-
Glutathione peroxidase
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- GR:
-
Glutathione reductase
- TBARS:
-
Thiobarbituric acid reactive substances
- K el :
-
Elimination rate cinstatnt
- t 1/2 :
-
Half-life
- C max :
-
The maximum (or peak) serum/plasma/brain concentration
References
Mackay J, Mensah G (2004) Atlas of heart disease and stroke. World Health Organization, Geneva, p 112E
Raza SS, Khan MM, Ashafaq M, Ahmad A, Khuwaja G, Khan A, Siddiqui MS, Safhi MM, Islam F (2011) Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: a behavioral, biochemical and immunohistological study in Wistar rats. J Neurol Sci 309(1–2):45–54
Takemura G, Onodera T, Millard RW, Ashraf M (1993) Demonstration of hydroxyl radical and its role in hydrogen peroxide-induced myocardial injury: hydroxyl radical dependent and independent mechanisms. Free Radic Biol Med 15:13–25
Hangaishi M, Nakajima H, Taguchi J, Igarashi R, Hoshino J, Kurokawa K, Kimura S, Nagai R, Ohno M (2001) Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia–reperfusion injury in rat hearts in vivo. Biochem Biophys Res Commun 285:1220–1225
Yang Z, Weian C, Susu H, Hanmin W (2016) Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol 771:145–151
Liu T, Song Y, Hu A (2021) Neuroprotective mechanisms of mangiferin in neurodegenerative diseases. Drug Dev Res 82(4):494–502
Du S, Liu H, Lei T, Xie X, Wang H, He X, Tong R, Wang Y (2018) Mangiferin: an effective therapeutic agent against several disorders (Review). Mol Med Rep 18(6):4775–4786
Xi J, Wang Y, Long X, Ma Y (2018) Mangiferin potentiates neuroprotection by isoflurane in neonatal hypoxic brain injury by reducing oxidative stress and activation of phosphatidylinositol-3-Kinase/Akt/Mammalian target of rapamycin (PI3K/Akt/mTOR) signaling. Med Sci Monit 24:7459–7468
Chen M, Wang Z, Zhou W, Lu C, Ji T, Yang W, Jin Z, Tian Y, Lei W, Wu S, Fu Q, Wu Z, Wu X, Han M, Fang M, Yang Y (2021) SIRT1/PGC-1α signaling activation by mangiferin attenuates cerebral hypoxia/reoxygenation injury in neuroblastoma cells. Eur J Pharmacol 907:174236
Bhatia HS, Candelario-Jalil E, de Oliveira ACP, Olajide OA, Martínez-Sánchez G, Fiebich BL (2008) Mangiferin inhibits cyclooxygenase-2 expression and prostaglandin E2 production in activated rat microglial cells. Arch Biochem Biophys 477:253–258
Das S, Rao BN, Rao BSS (2011) Mangiferin attenuates methylmercury induced cytotoxicity against IMR-32, human neuroblastoma cells by the inhibition of oxidative stress and free radical scavenging potential. Chem Biol Interact 193(2):129–140
Kasbe P, Jangra A, Lahkar M (2015) Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J Trace Elem Med Biol 31:107–112
Feng G, Zhang Z, Bao Q, Zhang Z, Zhou L, Jiang J, Li S (2014) Protective effect of chinonin in MPTP-induced C57BL/6 mouse model of Parkinson’s disease. Biol Pharm Bull 37(8):1301–1307
Kavitha M, Nataraj J, Essa MM, Memon MA, Manivasagam T (2013) Mangiferin attenuates MPTP induced dopaminergic neurodegeneration and improves motor impairment, redox balance and Bcl-2/Bax expression in experimental Parkinson’s disease mice. Chem Biol Interact 206(2):239–247
Ghosh M, Das J, Sil PC (2012) D(+) galactosamine induced oxidative and nitrosative stress-mediated renal damage in rats via NF-kappa B and inducible nitric oxide synthase (iNOS) pathways is ameliorated by a polyphenol xanthone, Mangiferin. Free Radic Res 46(2):116–132
Alkholifi FK, Alam A, Foudah AI, Yusufoglu HS (2023) Phospholipid-based topical nano-hydrogel of Mangiferin: enhanced topical delivery and improved dermatokinetics. Gels 9(3):178
Ahmad N, Al-Ghamdi MJA, Alnajjad HSM, Al Omar BBA, Khan MF, Al Malki ZS, Al Bassam AA, Ullah Z, Khalid MS, Ashraf K (2022) A comparative brain toxico-pharmacokinetics study of a developed tannic acid nanoparticles in the treatment of epilepsy. J Drug Deliv Sci Technol 76:103772
Selles AJN, Daglia M, Rastrelli L (2016) The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. Biofactors 42:475–491
Liu M, Liu Y, Ge Y, Zhong Z, Wang Z, Wu T, Zhao X, Zu Y (2020) Solubility, antioxidation, and oral bioavailability improvement of Mangiferin microparticles prepared using the supercritical antisolvent method. Pharmaceutics 12(2):90
Harsha P, Thotakura N, Kumar M, Sharma S, Mittal A, Khurana RK, Singh B, Negi P, Raza K (2019) A novel PEGylated carbon nanotube conjugated mangiferin: an explorative nanomedicine for brain cancer cells. J Drug Deliv Sci Technol 53:101186
Mao X, Cheng R, Zhang H, Bae J, Cheng L, Zhang L, Deng L, Cui W, Zhang Y, Santos HA, Sun X (2018) Self-healing and injectable hydrogel for matching skin flap regeneration. Adv Sci 6(3):1801555
Khurana RK, Gaspar BL, Welsby G, Katare OP, Singh KK, Singh B (2018) Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv Transl Res 8(3):617–632
Santonocito D, Vivero-Lopez M, Lauro MR, Torrisi C, Castelli F, Sarpietro MG, Puglia C (2022) Design of Nanotechnological carriers for ocular delivery of Mangiferin: preformulation study. Molecules 27(4):1328
Pires A, Fortuna A, Alves G, Falc˜ao A (2009) Intranasal drug delivery: how, why and what for? J Pharm Pharmaceut Sci 12(3):288–311
Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2(1):3–14
Wang CX, Huang LS, Hou LB, Jiang L, Yan ZT, Wang YL, Chen ZL (2009) Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res 1261:91–99
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces 75(1):1–18
Sempf K, Arrey T, Gelperina S, Schorge T, Meyer B, Karas M, Kreuter J (2013) Adsorption of plasma proteins on uncoated PLGA nanoparticles. Eur J Pharm Biopharm 85(1):53–60
Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R (2002) Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target 10(4):317–325
Pardridge WM (2006) Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol 6:494–500
Khan AR, Liu M, Khan MW, Zhai G (2017) Progress in brain targeting drug delivery system by nasal route. J Control Release 268:364–389
Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J (1999) Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res 16(10):1564–1569
Wilson B, Samanta MK, Santhi K, Kumar KPS, Paramakrishnan N, Suresh B (2008) Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur J Pharm Biopharm 70(1):75–84
Wohlfart S, Khalansky AS, Gelperina S, Begley D, Kreuter J (2011) Kinetics of transport of doxorubicin bound to nanoparticles across the blood-brain barrier. J Control Release 154(1):103–107
Yuan ZY, Hu YL, Gao JQ (2015) Brain localization and neurotoxicity evaluation of polysorbate 80-modified chitosan nanoparticles in rats. PLoS ONE 10(8):e0134722
Yusuf M, Khan M, Alrobaian MM, Alghamdi SA, Warsi MH, Sultana S, Khan RA (2021) Brain targeted polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J Drug Deliv Sci Technol 61:102214
Lindner GR, Santos DB, Colle D, Moreira ELG, Prediger RD, Farina M, Khalil NM, Mainardes RM (2015) Improved neuroprotective effects of resveratrol-loaded polysorbate 80-coated poly(lactide) nanoparticles in MPTP-induced Parkinsonism. Nanomedicine (Lond) 10(7):1127–1138
Ruan Y, Yao L, Zhang B, Zhang S, Guo J (2011) Antinociceptive properties of nasal delivery of neurotoxin-loaded nanoparticles coated with polysorbate-80. Peptides 32(7):1526–1529
Ruan Y, Yao L, Zhang B, Zhang S, Guo J (2012) Nanoparticle-mediated delivery of neurotoxin-II to the brain with intranasal administration: an effective strategy to improve antinociceptive activity of neurotoxin. Drug Dev Ind Pharm 38(1):123–128
Kaliappan I, Kammalla AK, Ramasamy MK, Aruna A, Dubey GP (2014) LC-MS quantification of Mangiferin inhydroalcoholic extract of Salacia oblonga, Salacia roxburghii and polyherbal formulation. Int J Phytopharm 4(1):11–15
Jong TT, Lee MR, Chiang YC, Chiang ST (2006) Using LC/MS/MS to determine matrine, oxymatrine, ferulic acid, mangiferin, and glycyrrhizin in the Chinese medicinal preparations Shiau-feng-saan and Dang-guei-nian-tong-tang. J Pharm Biomed Anal 40(2):472–477
Amir M, Ahmad N, Alqarni FSM, Sarafroz M (2020) A new UPLC method for the analysis of Mangiferin in Mangifera indica, Swertia chirayita and Canscora decussate by using fractional factorial design and its DPPH scavenging activity. Biosci Res 17(4):2614–2626
Imran M, Butt MS, Akhtar S, Riaz M, Iqbal MJ, Suleria HAR (2015) Quantification of Mangiferin by high pressure liquid chromatography; physicochemical and sensory evaluation of functional Mangiferin drink. J Food Process Preserv 40(4):760–769
Cai F, Sun L, Gao S, Zhan Q, Wang W, Chen W (2014) An improved LC-MS/MS method for the determination of mangiferin in rat plasma and its application in nonlinear pharmacokinetics. Pharmazie 69:168–172
Kammalla AK, Ramasamy MK, Inampudi J, Dubey GP, Agrawal A, Kaliappan I (2015) Comparative pharmacokinetic study of Mangiferin after oral administration of pure Mangiferin and US patented polyherbal formulation to rats. AAPS PharmSciTech 16(2):250–258
Guo H, Chen M, Li M, Hu M, Chen B, Zhou C (2019) Pharmacokinetic comparisons of Mangiferin and Mangiferin monosodium salt in rat plasma by UPLC-MS/MS. J Chem 2019:1–12
Qiu X, Zhao JL, Hao C, Yuan C, Tian N, Xu ZS, Zou RM (2016) Simultaneous determination of mangiferin and neomangiferin in rat plasma by UPLC-MS/MS and its application for pharmacokinetic study. J Pharm Biomed Anal 124:138–142
Sun D, Xue A, Zhang B, Lou H, Shi H, Zhang X (2015) Polysorbate 80-coated PLGA nanoparticles improve the permeability of acetyl puerarin and enhance its brain-protective effects in rats. J Pharm Pharmacol 67(12):1650–1662
McCall RL, Sirianni RW (2013) PLGA nanoparticles formed by single-or double emulsion with vitamin E-TPGS. JoVE J Vis Exp 82:e51015. https://doi.org/10.3791/51015
Ahmad N, Khalid MS, Khan MF, Ullah Z (2023) Beneficial effects of topical 6-gingerol loaded nanoemulsion gel for wound and inflammation management with their comparative dermatokinetic. J Drug Deliv Sci Technol 80:104094
US FDA (2001) Guidance for industry bioanalytical method validation. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. Accessed 24 May 2018
Ahmad N, Khalid MS, Al Ramadhan AM, Alaradi MZ, Al Hammad MR, Ansari K, Alqurashi YD, Khan MF, Albassam AA, Ansari MJ, Akhtar S, Dilshad M (2023) Preparation of melatonin novel-mucoadhesive nanoemulsion used in the treatment of depression. Polym Bull 80:8093–8132
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18(9):3470–3479
Ahmad N, Ahmad R, Amir M, Alam MA, Ali A, Ahmad A, Kamran A (2021) Ischemic brain treated with 6-gingerol loaded mucoadhesive nanoemulsion via intranasal delivery and their comparative pharmacokinetic effect in brain. J Drug Deliv Sci Technol 61:102130
Vaibhav K, Shrivastava P, Javed H, Khan A, Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Siddiqui MS, Safhi MM, Islam F (2012) Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol Cell Biochem 367(1–2):73–84
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol 33:1801–1807
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Youns M, Hoheisel JD, Efferth T (2011) Therapeutic and diagnostic applications of nanoparticles. Curr Drug Targets 12(3):357–365
Shmool TA, Zeitler JA (2019) Insights into the structural dynamics of poly lactic-coglycolic acid at terahertz frequencies. Polym Chem 10:351–361
Kamaly N, Yameen B, Wu J, Farokhzad OC (2016) Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev 116(4):2602–2663
Fredenberg S, Wahlgren M, Reslow M, Axelsson A (2011) The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm 415(1–2):34–52
Mehanny M, Hathout RM, Geneidi AS, Mansour S (2017) Studying the effect of physically-adsorbed coating polymers on the cytotoxic activity of optimized bisdemethoxycurcumin loaded-PLGA nanoparticles. J Biomed Mater Res 105(5):1433–1445
Zhang Z, Feng SS (2006) In vitro investigation on poly (lactide)–tween 80 copolymer nanoparticles fabricated by dialysis method for chemotherapy. Biomacromolecules 7(4):1139–1146
Fahmy HM, Khadrawy YA, Daim TMAE, Elfeky AS, Rabo AAA, Mustafa AB, Mostafa IT (2020) Thymoquinone-encapsulated chitosan nanoparticles coated with polysorbate 80 as a novel treatment agent in a reserpine-induced depression animal model. Physiol Behav 222:112934
Watrous-Peltier N, Uhl J, Steel V, Brophy L, Merisko-Liversidge E (1992) Direct suppression of phagocytosis by amphipathic polymeric surfactants. Pharm Res 9(9):1177–1183
Pamunuwa G, Karunaratne V, Karunaratne D (2016) Effect of lipid composition on in vitro release and skin deposition of curcumin encapsulated liposomes. J Nanomater 2016(35):4535790
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ (1997) Novel hydrophilic chitosan–polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci 63(1):125–132
Gao M, Mei D, Huo Y, Mao S (2019) Effect of polysorbate 80 on the intranasal absorption and brain distribution of tetramethylpyrazine phosphate in rats. Drug Deliv Transl Res 9(1):311–318
Som I, Bhatia K, Yasir M (2012) Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioall Sci 4(1):2–9
Kaur G, Mehta SK (2017) Developments of polysorbate (Tween) based microemulsions: preclinical drug delivery, toxicity and antimicrobial applications. Int J Pharm 529(1–2):134–160
Buyukozturk F, Benneyan JC, Carrier RL (2010) Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J Control Release 142(1):22–30
Levy G, Miller KE, Reuning RH (1966) Effect of complex formation on drug absorption III: concentration- and drug-dependent effect of a nonionic surfactant. J Pharm Sci 55(4):394–398
Ahmad N, Umar S, Ashafaq M (2013) A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma 250(6):1327–1338
Bolay H, Dalkara T (1998) Mechanisms of motor dysfunction after transient MCA occlusion: persistent transmission failure in cortical synapses is a major determinant. Stroke 29(9):1988–1993
Fukui K, Omoi NO, Hayasaka T, Shinnkai T, Suzuki S, Abe K, Urano S (2002) Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci 959:275–284
Hunter AJ, Mackay KB, Rogers DC (1998) To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents? Trends Pharmacol Sci 19(2):59–66
Zhan C, Yang J (2006) Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol Res 53(3):303–309
Funding
Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia have supported financially to conduct this study (PSAU/2023/R/1444).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
No conflict of the authors exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, N., Khan, M.F., Ullah, Z. et al. Development and evaluation of polysorbate-80 coated Mangiferin PLGA nanoparticles used in the treatment of cerebral ischemia. Polym. Bull. 81, 7035–7069 (2024). https://doi.org/10.1007/s00289-023-05030-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-05030-x