Abstract
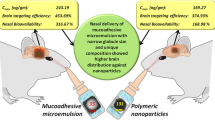
NMDAR (N-methyl-D-aspartate receptor) antagonist and opioid agonist can induce psychosis in rodents and humans. Loperamide hydrochloride (LH) possesses NMDAR antagonistic and opioid agonistic property but associated with more peripheral effects and limited blood brain barrier (BBB) penetration. So, it is essential to make its nanoformulation to increase BBB penetration and reduce peripheral effects. Thus, this study aimed to develop of LH encapsulated tween 80 coated chitosan nanoformulations and to optimize the formulations for targeting into brain. 32 full factorial design was performed to optimize the preparation to enhance the entrapment of the drug and reduce the particle size of all batches were between 189–278 nm and 80.16–89.38%, respectively. Pure drug LH (1 mg/kg, p.o) and LH loaded tween 80 coating chitosan nanoparticles (LCNP) (dose equal to 1 mg/kg LH, p.o.) were administrated in different group of animals to produce psychosis like behaviors in terms of stereotypic behaviors, hyper-locomotors activity, immobility and increased step down latency. LH and LCNP were administered orally for 15 successive days and behavioral, biochemical and histopathological alterations were studies. In mice, LCNP administration showed to induced behavioral changes similar to psychotic symptoms. However, administration of LH didn’t shows significant effect in cognitive symptom. Moreover, LCNP significantly decreased GSH and GABA level and increased MDA. Histopathologically, LCNP was significant to produce perinuclear vacuolization and hyperchromatic nuclei in the brain cortex, which indicates its damaging effects on brain. Thus, the surface modified NPs (LCNP) enhanced the penetration efficacy of LH. These studies revealed that LCNP could be effective chemical model to induce psychosis with good face, predictive and construct validity.













Similar content being viewed by others
Availability of data and material
Samples are available upon request from the initial author.
References
Chatterjee M, Verma R, Ganguly S, Palit G (2012) Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacol 63:1161–1171
Bubenikova-Valesova V, Horacek J, Vrajova M (2008) Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev 32:1014–1023
Kumar A, Yadav M, Parle P, Dhingra S, Dhull DK (2017) Potential drug targets and treatment of schizophrenia. Inflammopharmacol 25:277–292
Todjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D (2007) Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet 37:61–78
Parle M, Rana T (2012) How to test medicines of obsessive compulsive disorder. Int Res J Pharm 3(12):1–6
Liu X, Li J, Guo C, Wang H, Sun Y, Wang H, Su YA, Li K, Si T (2018) Olanzapine reverses MK-801-induced cognitive deficits and region-specific alterations of NMDA receptor subunits. Front Behav Neurosci 11:1–11
Upadhyay A, Bodar V, Malekzadegan M, Singh S, Frumkin W, Mangla A, Doshi K (2016) Loperamide induced life threatening ventricular arrhythmia. Case Rep Cardiol 2016:1–3
Nagpal K, Singh SK, Mishra DN (2013) Drug targeting to brain: a systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert Opin Drug Deliv 10:927–955
Hansraj GP, Singh SK, Kumar P (2015) Sumatriptan succinate loaded chitosan solid lipid NPs for enhanced anti-migraine potential. Int J Biol Macromol 81:467–476
Nagpal K, Singh SK, Mishra DN (2013) Evaluation of safety and efficacy of brain targeted chitosan NPs of minocycline. Int J Biol Macromol 59:20–28
Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni JK, Ali J (2012) Development and evaluation of rivastigmine loaded chitosan NPs for brain targeting. Eur J Pharm Sci 47:6–15
Guo F, Zhang M, Gao Y, Zhu S, Chen S, Liu W, Zhong H, Liu J (2016) Modified NPs with cell-penetrating peptide and amphipathic chitosan derivative for enhanced oral colon absorption of insulin: preparation and evaluation. Drug Deliv 23:2003–2014
Yadav M, Parle M, Sharma N, Dhingra S, Raina N, Jindal DK (2017) Brain targeted oral delivery of doxycycline hydrochloride encapsulated Tween 80 coated chitosan NPs against ketamine induced psychosis: behavioral, biochemical, neurochemical and histological alterations in mice. Drug Deliv 24:1429–1440
Nagpal K, Singh SK, Mishra DN (2013) Optimization of brain targeted gallic acid NPs for improved antianxiety-like activity. Int J Biol Macromol 57:83–91
Kandi P, Hayslett RL (2011) Nicotine and 17β-estradiol produce an antidepressant-like effect in female ovariectomized rats. Brain Res Bull 84:224–228
da Silva FCC, de Oliveira Cito MDC, da Silva MIG, Moura BA, de Aquino MR, Neto MR, Feitosa ML, de Castro Chaves R, Macedo DS, de Vasconcelos SMM, de França Fonteles MM, de Sousa FCF (2010) Behavioural alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res Bull 83:9–15
Sharma K, Parle M, Yadav M (2016) Evaluation of antipsychotic effect of methanolic extract of Ocimum sanctum leaves on laboratory animals. J Appl Pharm Sci 6:171–177
Chillar R, Dhingra D (2013) Anti-depressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fundam Clin Pharmacol 27:409–418
Soni K, Parle M (2017) Trachyspermumammi seeds supplementation helps reverse scopolamine, alprazolam and electroshock induced amnesia. Neurochem Res 42:1333–1344
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Wills ED (1964) The effect of inorganic iron on the thiobarbituric acid method for the determination of lipid peroxides. Biochem Biophys Acta 84:475–477
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Schlumpf M, Lichtensteiger W, Langemann H, Waser PG, Hefti F (1974) A fluorimetricmicromethod for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amount of brain tissue. Biochem Pharmacol 23:2437–2446
Lowe IP, Robins E, Eyerman GS (1958) The fluorimetric measurement of glutamic, decarboxylase measurement and its distribution in brain. J Neuro Chem 3:8–18
Tinku MM, Ahad A, Aqil M, Siddiqui WA, Najmi AK, Akhtar M, Shrivastava A, Qadir A, Moolakkadath T (2021) Ameliorative effect of rubiadinloaded nanocarriers in STZ-induced diabetic nephropathy in rats: formulation optimization, molecular docking, and in vivo biological evaluation. Drug Deliv Transl Res 12:615–628
Kreuter J (2001) Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 47:65–81
Zugno AI, Fraga DB, De Luca RD, Ghedim FV, Deroza PF, Cipriano AL, Oliveira MB, Heylmann AS, Budni J, Souza RP, Quevedo J (2013) Chronic exposure to cigarette smoke during gestation results in altered cholinesterase enzyme activity and behavioral deficits in adult rat offspring: potential relevance to schizophrenia. J Psychiatr Res 47:740–746
Acknowledgements
Authors would like to thank Guru Jambheshwar University of Science and Technology, Hisar, Haryana to provide financial assistance for this research work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MP gave the idea of research and supervised the work. MY, DKJ and NS did research work and wrote the manuscript. TG and DKJ have drawn tables and figures. KJ and NR revised the manuscript and polished the language. MY, NR and KJ revised the full manuscript. The final version of the manuscript has been approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, M., Parle, M., Sharma, N. et al. Development and evaluation of loperamide hydrochloride loaded chitosan nanoformulation for neurotoxic effects on mice. Polym. Bull. 81, 5111–5133 (2024). https://doi.org/10.1007/s00289-023-04952-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04952-w