Abstract
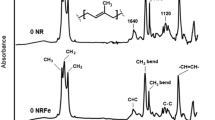
Liquefaction of vulcanized natural rubber presents a high value-added way out to the challenge of ever-increasing waste rubber. In this work, accelerated liquefaction of vulcanized natural rubber (NR) into liquid rubber through thermo-oxidative degradation was carried out at 210 ℃ in 15 min. The structural evolution of degraded NR was characterized by attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), elemental analysis (EA) and X-ray photoelectron spectroscopy (XPS). The results showed that vulcanized NR underwent a rapid process of oxygen absorption at the initial stage of degradation, which led to fast liquefaction. It was also illustrated by more oxygen-containing functional groups (–S=O and –C=O) increased with time in the liquid rubber. The Mn of the sol part sharply decreased to 1.259 \(\times\) 104 g mol−1 in 15 min. The volatile gases generated in the degradation process were characterized by thermogravimetric analysis coupled with Fourier transform infrared spectroscopy and mass spectrometry. These results indicated that the liquid rubber was obtained by efficient oxidative scission of the cross-linked sulfur bonds and the main chains. A liquefaction mechanism of vulcanized NR was proposed, which was also confirmed by density functional theory (DFT) calculations. This study provides a promising efficient approach to liquefy waste NR into a raw material for the recycling circle of rubber.









Similar content being viewed by others
References
Masatoshi Tosaka DK, Senoo K, Kohjiya S (2006) Crystallization and stress relaxation in highly stretched samples of natural rubber and its synthetic analogue. Macromolecules 39:5100–5105. https://doi.org/10.1021/ma060407+
Cao L, Sinha TK, Tao L, Li H, Zong C, Kim JK (2019) Synergistic reinforcement of silanized silica-graphene oxide hybrid in natural rubber for tire-tread fabrication: a latex based facile approach. Compos Part B- Eng 161:667–676. https://doi.org/10.1016/j.compositesb.2019.01.024
Dejchanchaiwong R, Kumar A, Tekasakul P (2019) Performance and economic analysis of natural convection based rubber smoking room for rubber cooperatives in Thailand. Renew Energ 132:233–242. https://doi.org/10.1016/j.renene.2018.07.145
Molino A, Donatelli A, Marino T, Aloise A, Rimauro J, Iovane P (2018) Waste tire recycling process for production of steam activated carbon in a pilot plant. Resour Conserv Recy 129:102–111. https://doi.org/10.1016/j.resconrec.2017.10.023
Fernández-Berridi MJ, González N, Mugica A, Bernicot C (2006) Pyrolysis-FTIR and TGA techniques as tools in the characterization of blends of natural rubber and SBR. Thermochim Acta 444(1):65–70. https://doi.org/10.1016/j.tca.2006.02.027
Asaro L, Gratton M, Seghar S, Aït Hocine N (2018) Recycling of rubber wastes by devulcanization. Resour Conserv Recy 133:250–262. https://doi.org/10.1016/j.resconrec.2018.02.016
Rajan VV, Dierkes WK, Joseph R, Noordermeer JWM (2006) Science and technology of rubber reclamation with special attention to NR-based waste latex products. Prog Polym Sci 31(9):811–834. https://doi.org/10.1016/j.progpolymsci.2006.08.003
Kojima M, Tosaka M, Ikeda Y (2004) Chemical recycling of sulfur-cured natural rubber using supercritical carbon dioxide. Green Chem 6(2):84–89. https://doi.org/10.1039/b314137c
Zhang XX, Lu CH, Liang M (2013) Devulcanisation of natural rubber vulcanisate through solid state mechanochemical milling at ambient temperature. Plasti Rubber Compos 36(7–8):370–376. https://doi.org/10.1179/174328907x237584
Ghorai S, Bhunia S, Roy M, De D (2016) Mechanochemical devulcanization of natural rubber vulcanizate by dual function disulfide chemicals. Polym Degrad Stabil 129:34–46. https://doi.org/10.1016/j.polymdegradstab.2016.03.024
Shi J, Zou H, Ding L, Li X, Jiang K, Chen T, Zhang X, Zhang L, Ren D (2014) Continuous production of liquid reclaimed rubber from ground tire rubber and its application as reactive polymeric plasticizer. Polym Degrad Stabil 99:166–175. https://doi.org/10.1016/j.polymdegradstab.2013.11.010
Sutanto P, Laksmana FL, Picchioni F, Janssen LPBM (2006) Modeling on the kinetics of an EPDM devulcanization in an internal batch mixer using an amine as the devulcanizing agent. Chem Eng Sci 61(19):6442–6453. https://doi.org/10.1016/j.ces.2006.05.024
Rattanasom N, Poonsuk A, Makmoon T (2005) Effect of curing system on the mechanical properties and heat aging resistance of natural rubber/tire tread reclaimed rubber blends. Polym Test 24(6):728–732. https://doi.org/10.1016/j.polymertesting.2005.04.008
Ibrahim S, Daik R, Abdullah I (2014) Functionalization of liquid natural rubber via oxidative degradation of natural rubber. Polymers 6(12):2928–2941. https://doi.org/10.3390/polym6122928
Martínez A, Tlenkopatchev MA, Gutiérrez S, Burelo M, Vargas J, Jiménez-Regalado E (2019) Synthesis of unsaturated esters by cross-metathesis of terpenes and natural rubber using Ru-alkylidene catalysts. Curr Org Chem 23(12):1356–1364. https://doi.org/10.2174/1385272823666190723125427
Burelo M, Martínez A, Cruz-Morales JA, Tlenkopatchev MA, Gutiérrez S (2019) Metathesis reaction from bio-based resources: Synthesis of diols and macrodiols using fatty alcohols, β-citronellol and natural rubber. Polym Degrad Stabil 166:202–212. https://doi.org/10.1016/j.polymdegradstab.2019.05.021
Tripathy AR, Morin JE, Williams DE, Eyles SJ, Farris RJ (2002) A novel approach to improving the mechanical properties in recycled vulcanized natural rubber and its mechanism. Macromolecules 35(12):4616–4627. https://doi.org/10.1021/ma012110b
Tripathy AR, Williams DE, Farris RJ (2004) Rubber plasticizers from degraded/devulcanized scrap rubber: A method of recycling waste rubber. Polym Eng Sci 44(7):1338–1350. https://doi.org/10.1002/pen.20129
Wu X, Wang S, Dong R (2016) Lightly pyrolyzed tire rubber used as potential asphalt alternative. Constr Build Mater 112:623–628. https://doi.org/10.1016/j.conbuildmat.2016.02.208
Grasland F, Chazeau L, Chenal J-M, schach R, (2019) About thermo-oxidative ageing at moderate temperature of conventionally vulcanized natural rubber. Polym Degrad Stabil 161:74–84. https://doi.org/10.1016/j.polymdegradstab.2018.12.029
Danielle Galiani P, Antonio Malmonge J, Guenther Soares B, Henrique Capparelli Mattoso L (2013) Studies on thermal–oxidative degradation behaviours of raw natural rubber: PRI and thermogravimetry analysis. Plasti Rubber Compos 42(8):334–339. https://doi.org/10.1179/1743289811y.0000000046
Shelton J (1972) Review of basic oxidation processes in elastomers rubber. Chem Technol 45(2):359–380
Pazur RJ, Petrov I (2015) The thermo-oxidation of chlorinated and brominated isobutylene-co-isoprene polymers: activation energies and reactions from room temperature to 100 °C. Polym Degrad Stabil 121:311–320. https://doi.org/10.1016/j.polymdegradstab.2015.09.023
Xie Y, Hassan AA, Song P, Zhang Z, Wang S (2019) High scission of butadiene rubber vulcanizate under thermo-oxidation. Polym Degrad Stabil 167:292–301. https://doi.org/10.1016/j.polymdegradstab.2019.07.015
Song P, Wan C, Xie Y, Formela K, Wang S (2018) Vegetable derived-oil facilitating carbon black migration from waste tire rubbers and its reinforcement effect. Waste Manage 78:238–248. https://doi.org/10.1016/j.wasman.2018.05.054
Zhao Y, Truhlar DG (2007) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120(1–3):215–241. https://doi.org/10.1007/s00214-007-0310-x
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE et al (2016) Gaussian 16 Rev B01. Wallingford, CT
Maeda S, Harabuchi Y, Ono Y, Taketsugu T, Morokuma K (2015) Intrinsic reaction coordinate: calculation, bifurcation and automated search. Int J Quantum Chem 115(5):258–269. https://doi.org/10.1002/qua.24757
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
William Humphrey AD, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Horikx MM (1956) Chain scissions in a polymer network. J Polym Sci 59:445–454
Zheng W, Liu L, Zhao X, He J, Wang A, Chan TW, Wu S (2015) Data for effects of lanthanum complex on the thermo-oxidative aging of natural rubber. Data Brief 5:789–795. https://doi.org/10.1016/j.dib.2015.10.032
Aoudia K, Azem S, Ait Hocine N, Gratton M, Pettarin V, Seghar S (2017) Recycling of waste tire rubber: microwave devulcanization and incorporation in a thermoset resin. Waste Manag 60:471–481. https://doi.org/10.1016/j.wasman.2016.10.051
Nabil H, Ismail H, Azura AR (2013) Comparison of thermo-oxidative ageing and thermal analysis of carbon black-filled NR/Virgin EPDM and NR/Recycled EPDM blends. Polym Test 32(4):631–639. https://doi.org/10.1016/j.polymertesting.2013.03.019
Xu F, Wang B, Yang D, Ming X, Jiang Y, Hao J, Qiao Y, Tian Y (2018) TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energ Convers Manage 175:288–297. https://doi.org/10.1016/j.enconman.2018.09.013
Hu H, Fang Y, Liu H, Yu R, Luo G, Liu W, Li A, Yao H (2014) The fate of sulfur during rapid pyrolysis of scrap tires. Chemosphere 97:102–107. https://doi.org/10.1016/j.chemosphere.2013.10.037
Formela K, Klein M, Colom X, Saeb MR (2016) Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber (GTR). Polym Degrad Stabil 125:1–11. https://doi.org/10.1016/j.polymdegradstab.2015.12.022
Li S, Wan C, Wu X, Wang S (2016) Core-shell structured carbon nanoparticles derived from light pyrolysis of waste tires. Polym Degrad Stabil 129:192–198. https://doi.org/10.1016/j.polymdegradstab.2016.04.013
Modrow HZ, ViselHormes FJ (2000) Monitoring thermal oxidation of sulfur crosslinks in SBRElastomers using Sulfur K-edge XANES: a feasibility study. KGK Kaut Gummi Kunst 53(6):328–337
Acknowledgements
The authors are grateful for the research foundation provided by International Cooperation Project by MOST, SQ2019YFE012146, Natural Science Foundation of China (No. 51273110) and the Jiangsu Science and Technology Project (BA2016002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Z., Zhang, Y., Li, J. et al. Accelerated liquefaction of vulcanized natural rubber by thermo-oxidative degradation. Polym. Bull. 79, 1767–1786 (2022). https://doi.org/10.1007/s00289-021-03580-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03580-6