Abstract
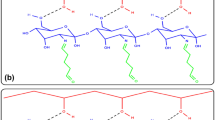
In this study, poly[methyl methacrylate-co-poly(ethylene glycol) methacrylate] (P(MMA-co-PEG500MA)) copolymers were used for catalase (CAT) immobilization. Firstly, P(MMA-co-PEG500MA) copolymers were synthesized by using different amount of methyl methacrylate (MMA) and poly(ethylene glycol) methacrylate (PEG500MA) monomers. The synthesized copolymers were characterized by different analysis techniques. Afterward, CAT enzyme was immobilized via physical adsorption method onto the P(MMA-co-PEG500MA) copolymers. P3 sample containing 1:1 (PEG500MA:MMA) monomer molar ratio was selected as model support because of exhibiting optimum surface porosity and thermal stability. A high immobilization yield (76%) was achieved under optimized conditions. The immobilized enzyme displayed improved tolerance towards pH and temperature changes. After immobilization, the optimum pH shifted from 7.5 to 7.0, whereas the optimum temperature remained unchanged at 35 °C. Immobilized enzyme showed good reuse potential and excellent storage stability. After 10 consecutive uses, immobilized enzyme maintained about 51.0% of its initial activity. Furthermore, free enzyme completely lost its initial activity after 4 weeks, while immobilized enzyme maintained approximately 65% of the initial activity at 25 °C. Approximately twofold decrease in Km was obtained which means that the affinity of enzyme to the substrate improved after immobilization. Finally, it can be concluded that the prepared P(MMA-co-PEG500MA) copolymer structure can be an ideal matrix for CAT immobilization.














Similar content being viewed by others
References
Koushal V, Sharma R, Sharma M, Sharma R, Sharma V (2014) Plastics: issues challenges and remediation. Int J Waste Resour 4(1):134
Raheem D (2012) Application of plastics and paper as food packaging materials—an overview. Emir J Food Agric 25(3):177–188
Mülhaupt R (2013) Green polymer chemistry and bio-based plastics: dreams and reality. Macromol Chem Phys 214:159–174
Ignatyev IA, Thielemans W, Vander Beke B (2014) Recycling of polymers: a review. ChemSusChem 7:1579–1593
Luzi F, Torre L, Kenny JM, Puglia D (2019) Bio- and fossil-based polymeric blends and nanocomposites for packaging: structure–property relationship. Materials (Basel) 12(3):471
Othman SH (2014) Bio-nanocomposite materials for food packaging applications: types of biopolymer and nano-sized filler. Agric Agric Sci Procedia 2:296–303
Fan B, Zhou M, Zhang C, He D, Bai J (2019) Polymer-based materials for achieving high energy density film capacitors. Prog Polym Sci 97:101143
Balakrishnan P, Geethamma VG, Sreekala MS, Thomas S (2018) Polymeric biomaterials: state-of-the-art and new challenges. In: Thomas S, Balakrishnan P, Sreekala MS (eds) Woodhead publishing series in biomaterials, fundamental biomaterials: polymers. Woodhead Publishing, Sawston, pp 1–20
Shah SA, Sohail M, Khan S, Minhas MU, de Matas M, Sikstone V, Hussain Z, Abbasi M, Kousar M (2019) Biopolymer-based biomaterials for accelerated diabetic wound healing: a critical review. Int J Biol Macromol 139:975–993
Abbasian M, Massoumi B, Mohammad-Rezaei R, Samadian H, Jaymand M (2019) Scaffolding polymeric biomaterials: are naturally occurring biological macromolecules more appropriate for tissue engineering? Int J Biol Macromol 134:673–694
Feng C, Huang X (2018) Polymer brushes: efficient synthesis and applications. Acc Chem Res 51(9):2314–2323
Advincula RC, Brittain WJ, Caster KC, Ruehe J (2004) Polymer brushes. Wiley-VCH, Weinheim
Milner ST (1991) Polymer brushes. Science 251:905–914
Zhao B, Brittain WJ (2000) Polymer brushes: surface-immobilized macromolecules. Prog Polym Sci 25:677–710
Bumbu GG, Kircher G, Wolkenhauer M, Berger R, Gutmann JS (2004) Synthesis and characterization of polymer brushes on micromechanical cantilevers. Macromol Chem Phys 205:1713–1720
Krishnamoorthy M, Hakobyan S, Ramstedt M, Gautrot JE (2014) Surface-initiated polymer brushes in the biomedical field: applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem Rev 114:10976–11026
Koenig M, König U, Eichhorn K-J, Müller M, Stamm M, Uhlmann P (2019) In-situ-investigation of enzyme immobilization on polymer brushes. Front Chem 7(101):1–8
Koenig M, Bittrich E, König U, Rajeev BL, Müller M, Eichhorn K-J, Thomas S, Stamm M, Uhlmann P (2016) Adsorption of enzymes to stimuli-responsive polymer brushes: influence of brush conformation on adsorbed amount and biocatalytic activity. Colloids Surf B Biointerfaces 146:737–745
del Castillo GF-D, Koenig M, Müller M, Eichhorn K-J, Stamm M, Uhlmann P, Dahlin A (2019) Enzyme immobilization in polyelectrolyte brushes: high loading and enhanced activity compared to monolayers. Langmuir 35:3479–3489
Xu FJ, Cai QJ, Li YL, Kang ET, Neoh KG (2005) Covalent immobilization of glucose oxidase on well-defined poly(glycidyl methacrylate)−Si(111) hybrids from surface-initiated atom-transfer radical polymerization. Biomacromolecules 6(2):1012–1020
Qin W, Song Z, Fan C, Zhang W, Cai Y, Zhang Y, Qian X (2012) Trypsin immobilization on hairy polymer chains hybrid magnetic nanoparticles for ultra fast, highly efficient proteome digestion, facile 18O labeling and absolute protein quantification. Anal Chem 847:3138–3144
Rafiee-Pour HA, Nejadhosseinian M, Firouzi M, Masoum S (2019) Catalase immobilization onto magnetic multi-walled carbon nanotubes: optimization of crucial parameters using response surface methodology. New J Chem 43:593–600
Arabaci G, Usluoglu A (2013) Catalytic properties and immobilization studies of catalase from Malva sylvestris L. J Chem 686185:1–6
Czechowska E, Ranoszek-Soliwoda K, Tomaszewska E, Pudlarz A, Celichowski G, Gralak-Zwolenik D, Szemraj J, Grobelny J (2018) Comparison of the antioxidant activity of catalase immobilized on gold nanoparticles via specific and non-specific adsorption. Colloids Surf B Biointerfaces 171:707–714
Zhang L, Sun Y (2018) Poly(carboxybetaine methacrylate)-grafted silica nanoparticle: a novel carrier for enzyme immobilization. Biochem Eng J 132:122–129
Costa SA, Reis RL (2004) Immobilisation of catalase on the surface of biodegradable starch-based polymers as a way to change its surface characteristics. J Mater Sci Mater Med 15(4):335–342
Kaushal J, Seema SG, Arya SK (2018) Immobilization of catalase onto chitosan and chitosan–bentonite complex: a comparative study. Biotechnol Rep 18(e00258):1–6
Inanan T (2019) Chitosan co-polymeric nanostructures for catalase immobilization. React Funct Polym 135:94–102
Feng Y, Zhong L, Bilal M, Tan Z, Hou Y, Jia S, Cui J (2018) Enzymes@ZIF-8 nanocomposites with protection nanocoating: stability and acid-resistant evaluation. Polymers (Basel) 11:1–14
Feng Q, Zhao Y, Wei A, Li C, Wei Q, Fong H (2014) Immobilization of catalase on electrospun PVA/PA6–Cu(II) nanofibrous membrane for the development of efficient and reusable enzyme membrane reactor. Environ Sci Technol 48(17):10390–10397
Bayramoglu G, Karagoz B, Yilmaz M, Bicak N, Arica MY (2011) Immobilization of catalase via adsorption on poly(styrene-d-glycidylmethacrylate) grafted and tetraethyldiethylenetriamine ligand attached microbeads. Bioresour Technol 102(4):3653–3661
Murtinho D, Lagoa AR, Garcia FAP, Gil MH (1998) Cellulose derivatives membranes as supports for immobilisation of enzymes. Cellulose 5:299–308
Airoldi C, Monteiro Junior O (2003) Copper adsorption and enzyme immobilization on organosilane-glutaraldehyde hybrids as support. Polym Bull 50:61–68
Ingenbosch KN, Rousek A, Wunschik DS, Hoffmann-Jacobsen K (2019) A fluorescence-based activity assay for immobilized lipases in non-native media. Anal Biochem 569:22–27
Cerqueira MRF, Santos MSF, Matos RC, Gutz IGR, Angnes L (2015) Use of poly(methyl methacrylate)/polyethyleneimine flow microreactors for enzyme immobilization. Microchem J 118:231–237
Li S, Hu J, Liu B (2004) Use of chemically modified PMMA microspheres for enzyme immobilization. Biosystems 77(1–3):25–32
Bulmuş V, Ayhan H, Pişkin E (1997) Modified PMMA monosize microbeads for glucose oxidase immobilization. Chem Eng J Biochem Eng J 65(1):71–76
Lai Y, Wang F, Zhang Y, Ou P, Wu P, Fang Q, Li S, Chen Z (2019) Effective removal of methylene blue and orange II by subsequent immobilized laccase decolorization on crosslinked polymethacrylate/carbon nanotubes. Mater Res Express 6(085541):1–11
Pugazhenthi G, Kumar A (2004) Enzyme membrane reactor for hydrolysis of olive oil using lipase immobilized on modified PMMA composite membrane. J Membr Sci 228:187–197
Ulu A, Koytepe S, Ates B (2016) Synthesis and characterization of PMMA composites activated with starch for immobilization of L-asparaginase. J Appl Polym Sci 43421:1–11
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Patil PD, Yadav GD (2018) Rapid in situ encapsulation of laccase into metal-organic framework support (ZIF-8) under biocompatible conditions. ChemistrySelect 3:4669–4675
Kanimozhi G, Vinoth S, Harish K, Srinadhu E, Satyanarayana N (2019) Conductivity and dielectric permittivity studies of KI-based nanocomposite (PEO/PMMA/KI/I2/ZnO nanorods) polymer solid electrolytes. Polym Compos 40:2919–2928
Abdelrazek E, Elashmawi I, Soliman M, Aly A (2009) Physical properties of MnCl2 fillers incorporated into a PVDF/PVC blend and their complexes. J Vinyl Addit Technol 15:171–177
Poomalai P, Varghese TO, Siddaramaiah (2011) Thermomechanical behaviour of poly(methylmethacrylate)/copoly(ether-ester) blends. Int Sch Res Netw ISRN Mater Sci 2011:1–5
Ramírez-Jiménez A, Montoya-Villegas KA, Licea-Claverie A, Gónzalez-Ayón MA (2019) Tunable thermo-responsive copolymers from DEGMA and OEGMA synthesized by RAFT polymerization and the effect of the concentration and saline phosphate buffer on its phase transition. Polymers 11:1657
Tsai B, Garcia-Valdez O, Champagne P, Cunningham MF (2017) Poly(poly(ethylene glycol) methyl ether methacrylate) grafted chitosan for dye removal from water. Processes 5(12):1–13
Asmat S, Husain Q, Azam A (2017) Lipase immobilization on facile synthesized polyaniline-coated silver-functionalized graphene oxide nanocomposites as novel biocatalysts: stability and activity insights. RSC Adv 7:5019–5029
Saleem M, Rafiq M, Seo SY, Lee KH (2016) Acetylcholinesterase immobilization and characterization, and comparison of the activity of the porous silicon-immobilized enzyme with its free counterpart. Biosci Rep 36(art:00311):1–11
Patel SKS, Otari SV, Chan Kang Y, Lee JK (2017) Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production. RSC Adv 7:3488–3494
Samui A, Sahu SK (2018) One-pot synthesis of microporous nanoscale metal organic frameworks conjugated with laccase as a promising biocatalyst. New J Chem 42:4192–4200
Çetinus ŞA, Öztop HN, Saraydin D (2007) Immobilization of catalase onto chitosan and cibacron blue F3GA attached chitosan beads. Enzyme Microb Technol 41(4):447–454
Erol K, Cebeci BK, Köse K, Köse DA (2019) Effect of immobilization on the activity of catalase carried by poly(HEMA-GMA) cryogels. Int J Biol Macromol 123:738–743
Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong SH, Kim T, Haw JR, Kim SY, Kim IW, Lee JK (2018) Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol 28(4):638–644
Sharma N (2013) Kinetic study of free and immobilized protease from Aspergillus sp. IOSR J Pharm Biol Sci 7(2):86–96
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sel, E., Ulu, A., Ateş, B. et al. Comparative study of catalase immobilization via adsorption on P(MMA-co-PEG500MA) structures as an effective polymer support. Polym. Bull. 78, 2663–2684 (2021). https://doi.org/10.1007/s00289-020-03233-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03233-0