Abstract
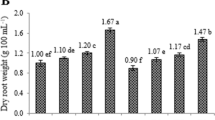
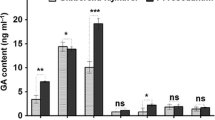
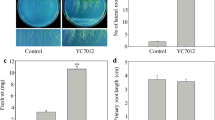
Endophytic fungi play important roles in regulating plant growth and development and usually used as a promising strategy to enhance the biosynthesis of host valuable secondary metabolite, but the underlying growth-promoting mechanisms are only partly understood. In this study, the wild-type Arabidopsis thaliana seedlings co-cultured with fungal endophyte Epichloë bromicola showed auxin (IAA)-stimulated phenotypes, and the growth-promoting effects caused by E. bromicola were further verified by the experiments of spatially separated co-culture and fungal extract treatment. IAA was detected and identified in the extract of E. bromicola culture by LC-HRMS/MS, whereas 2,3-butanediol was confirmed to be the predominant volatile active compound in the diethyl ether and ethyl acetate extracts by GC–MS. Further study observed that IAA-related genes including synthesis key enzyme genes (CYP79B2, CYP79B3, NIT1, TAA1 and YUCCA1) and controlling polar transport genes (AUX1, BIG, EIR1, AXR3 and ARF1), were highly expressed at different periods after E. bromicola inoculation. More importantly, the introduction of fungal endophyte E. bromicola could effectively promote the growth and accumulation of coixol in Coix under soil conditions. Our study showed that endophytic fungus E. bromicola might be considered as a potential inoculant for improving medicinal plant growth.





Similar content being viewed by others
Data Availability
The raw data in this article will be accessible by the authors without undue reservation.
Code Availability
Not applicable.
References
Omomowo OI, Babalola OO (2019) Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 7:481. https://doi.org/10.3390/microorganisms7110481
Poveda J, Eugui D, Abril-Urías P, Velasco P (2021) Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis 85:1–19. https://doi.org/10.1007/s13199-021-00789-x
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K et al (2016) A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol 7:906. https://doi.org/10.3389/fmicb.2016.00906
Zhai X, Luo D, Li X, Han T, Jia M et al (2018) Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front Microbiol 8:2694. https://doi.org/10.3389/fmicb.2017.02694
Gupta S, Chaturvedi P, Kulkarni MG, Van Staden J (2020) A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol Adv 39:107462. https://doi.org/10.1016/j.biotechadv.2019.107462
Lu H, Wei T, Lou H, Shu X, Chen Q (2021) A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J Fungi 7:719. https://doi.org/10.3390/jof7090719
Khalil AMA, Hassan SED, Alsharif SM, Eid AM, Ewais EED et al (2021) Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules 11:140. https://doi.org/10.3390/biom11020140
Zhang W, Sun K, Shi RH, Yuan J, Wang XJ et al (2018) Auxin signalling of Arachis hypogaea activated by colonization of mutualistic fungus Phomopsis liquidambari enhances nodulation and N2-fixation. Plant Cell Environ 41:2093–2108. https://doi.org/10.1111/pce.13170
Zhang W, Li XG, Sun K, Tang MJ, Xu FJ et al (2020) Mycelial network-mediated rhizobial dispersal enhances legume nodulation. ISME J 14:1015–1029. https://doi.org/10.1038/s41396-020-0587-5
Wu S, Xie X, Yang Y, Zheng C, Han T (2022) Effects of endophytic fungus SH09 on plant growth and accumulation of active components in Salvia miltiorrhiza. J Pharm Pract 40:213–217. https://doi.org/10.12206/j.issn.1006-0111.202108055
Zhou J, Li X, Huang PW, Dai CC (2018) Endophytism or saprophytism: decoding the lifestyle transition of the generalist fungus Phomopsis liquidambari. Microbiol Res 206:99–112. https://doi.org/10.1016/j.micres.2017.10.005
Zhou J, Huang PW, Li X, Vaistij FE, Dai CC (2022) Generalist endophyte Phomopsis liquidambaris colonization of Oryza sativa L. promotes plant growth under nitrogen starvation. Plant Mol Biol. https://doi.org/10.1007/s11103-022-01268-7
Song H, Nan Z, Song Q, Xia C, Li X et al (2016) Advances in research on Epichloë endophytes in Chinese native grasses. Front Microbiol 7:1399. https://doi.org/10.3389/fmicb.2016.01399
Song QY, Li F, Nan ZB, Coulter JA, Wei WJ (2020) Do Epichloë endophytes and their grass symbiosis only produce toxic alkaloids to insects and livestock? J Agr Food Chem 68:1169–1185. https://doi.org/10.1021/acs.jafc.9b06614
Chen T, Li C, White JF, Nan Z (2019) Effect of the fungal endophyte Epichloë bromicola on polyamines in wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 436:29–48. https://doi.org/10.1007/s11104-018-03913-x
Wang Z, Li C, White J (2019) Effects of Epichloë endophyte infection on growth, physiological properties and seed germination of wild barley under saline conditions. J Agron Crop Sci 206:43–51. https://doi.org/10.1111/jac.12366
Zipfel C, Oldroyd GE (2017) Plant signalling in symbiosis and immunity. Nature 543:328–336. https://doi.org/10.1038/nature22009
Kong HG, Shin TS, Kim TH, Ryu CM (2018) Stereoisomers of the bacterial volatile compound 2,3-butanediol differently elicit systemic defense responses of pepper against multiple viruses in the field. Front Plant Sci 9:90. https://doi.org/10.3389/fpls.2018.00090
Rajani P, Rajasekaran C, Vasanthakumari MM, Olsson SB, Ravikanth G et al (2021) Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol Res 242:126595. https://doi.org/10.1016/j.micres.2020.126595
Waqas M, Khan AL, Hamayun M, Shahzad R, Kang SM et al (2015) Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J Plant Interact 10:280–287. https://doi.org/10.1080/17429145.2015.1079743
Aslam MM, Karanja J, Bello SK (2019) Piriformospora indica colonization reprograms plants to improved P-uptake, enhanced crop performance, and biotic/abiotic stress tolerance. Physiol Mol Plant Pathol 106:232–237. https://doi.org/10.1016/j.pmpp.2019.02.010
Galeano RMS, Franco DG, Chaves PO, Giannesi GC, Masui DC et al (2021) Plant growth promoting potential of endophytic Aspergillus niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region, Brazil. Rhizosphere 18:100332. https://doi.org/10.1016/j.rhisph.2021.100332
Bei S, Xu M, Lyu X, Chen C, Li A et al (2021) Arbuscular mycorrhizal fungi enhanced coix responses to phosphorous forms but not for faba bean in intercropping systems, under controlled environment. Agron J 113:2578–2590. https://doi.org/10.1002/agj2.20643
Zhang YB, He XH, Liu FZ, Meng QY, Liu R et al (2019) Screening and analysis of germplasm resources of wild Coix lacryma-jobi L. with high content of coixin. Med Plant 10:66–68. https://doi.org/10.19600/j.cnki.issn2152-3924.2019.02.017
Feng L, Zhao Y, Zhang Z, Zhang S, Zhang H et al (2020) The edible and medicinal value of Coix lacryma-jobi and key cultivation techniques for high and stable yield. Nat Resour 11:569–575. https://doi.org/10.4236/nr.2020.1112034
Patel B, Patel G, Shah S, Parmar S (2017) A review: Coix lacryma jobi L. Res J Pharmacogn Phytochem 9:248–252. https://doi.org/10.5958/0975-4385.2017.00046.2
Wang L, Sui N, Lv H, Tang Q, Shi M et al (2022) Effects of potassium foliage supplementation on Coix lacryma-jobi L. yield formation and source-sink relationship compared with those of soil supplementation. Ind Crop Prod 180:114754. https://doi.org/10.1016/j.indcrop.2022.114754
Ren WY, Su J, Liu YQ, Hou WQ, Zheng YJ et al (2020) Analysis on diagnosis and treatment scheme of traditional Chinese medicine in treatment of COVID-19 in Chinese provinces and regions. Chin Trad Herb Drugs 24:1139–1146. https://doi.org/10.7501/j.issn.0253-2670.2020.05.007
Diao X (2017) Production and genetic improvement of minor cereals in China. Crop J 5:103–114. https://doi.org/10.1016/j.cj.2016.06.004
Miao G, Qin Y, Guo J, Zhang Q, Bao Y (2021) Transcriptome characterization and expression profile of Coix lacryma-jobi L. in response to drought. PLoS ONE 16:e0256875. https://doi.org/10.1371/journal.pone.0256875
Li GR, Cao BH, Liu W, Ren RH, Feng J et al (2020) Isolation and identification of endophytic fungi in kernels of Coix lachrymal-jobi L. cultivars. Curr Microbiol 77:1448–1456. https://doi.org/10.1007/s00284-020-01950-3
Jia M, Ming QL, Zhang QY, Chen Y, Cheng N et al (2014) Gibberella moniliformis AH13 with antitumor activity, an endophytic fungus strain producing triolein isolated from adlay (Coix lacryma-jobi: Poaceae). Curr Microbiol 69:381–387. https://doi.org/10.1007/s00284-014-0590-z
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S et al (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13:843–852. https://doi.org/10.1105/tpc.13.4.843
Han L, Zhou X, Zhao Y, Zhu S, Wu L et al (2020) Colonization of endophyte Acremonium sp. D212 in Panax notoginseng and rice mediated by auxin and jasmonic acid. J Integr Plant Biol 62:1433–1451. https://doi.org/10.1111/jipb.12905
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX et al (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932. https://doi.org/10.1073/pnas.0730845100
Al-Rashdi FKH, Al-Sadi AM, Al-Riyamy BZ, Maharachchikumbura SS, Al-Sabahi JN et al (2020) Endophytic fungi from the medicinal plant Aloe dhufarensis lavranos exhibit antagonistic potential against phytopathogenic fungi. S Afr J Bot 147:1078–1085. https://doi.org/10.1016/j.sajb.2020.05.022
Sun H, Zuo X, Zhang Q, Gao J, Kai G (2022) Elicitation of (E)-2-hexenal and 2,3-butanediol on the bioactive compounds in adventitious roots of Astragalus membranaceus var. mongholicus. J Agric Food Chem 70:470–479. https://doi.org/10.1021/acs.jafc.1c05813
Li TY, Ye C, Zhang YJ, Zhang JX, Yang M et al (2022) 2,3-butanediol from the leachates of pine needles induces the resistance of Panax notoginseng to the leaf pathogen Alternaria panax. Plant Divers. https://doi.org/10.1016/j.pld.2022.02.003
Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592. https://doi.org/10.1104/pp.108.130369
Felten J, Kohler A, Morin E, Bhalerao RP, Palme K et al (2009) The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 151:1991–2005. https://doi.org/10.1104/pp.109.147231
Sun X, Wang N, Li P, Jiang Z, Liu X et al (2020) Endophytic fungus Falciphora oryzae promotes lateral root growth by producing indole derivatives after sensing plant signals. Plant Cell Environ 43:358–373. https://doi.org/10.1111/pce.13667
Ye B, Wu Y, Zhai X, Zhang R, Wu J et al (2020) Beneficial effects of endophytic fungi from the Anoectochilus and Ludisia species on the growth and secondary metabolism of Anoectochilus roxburghii. ACS Omega 5:3487–3497. https://doi.org/10.1021/acsomega.9b03789
Acknowledgements
The authors are deeply grateful to the anonymous reviewers and editorial staff for their valuable time and attention.
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 82174091 and 81872953), the Basic Medical Research Project of Naval Medical University (No. 2022MS004), and the Undergraduate Innovation Incubation Base Project of Naval Medical University (No. FR2021112).
Author information
Authors and Affiliations
Contributions
LPQ, TH and CJZ conceived and designed the research; XGX and WLL performed the research, and wrote and revised manuscript; KMF and YY performed part of the experiments; MJ, YSW and YZS analyzed the data; all authors have read and approved the final manuscript. XGX and WLL contributed equally to this work and share first authorship.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, XG., Lu, WL., Feng, KM. et al. Mechanisms of Epichloë bromicola to Promote Plant Growth and Its Potential Application for Coix lacryma-jobi L. Cultivation. Curr Microbiol 80, 306 (2023). https://doi.org/10.1007/s00284-023-03411-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03411-z