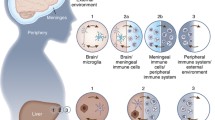
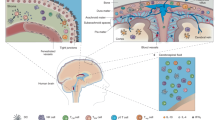
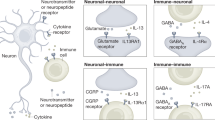
Abstract
The brain is an immune-privileged organ such that immune cell infiltration is highly regulated and better tolerating the introduction of antigen to reduce risk of harmful inflammation. Thus, the composition and the nature of the immune response is fundamentally different in the brain where avoiding immunopathology is prioritized compared to other peripheral organs. While the principle of immune privilege in the central nervous system (CNS) still holds true, the role of the immune system in the CNS has been revisited over the recent years. This redefining of immune privilege in the brain is a result of the recent re-discovery of the extensive CNS meningeal lymphatic system and the identification of resident T cells in the brain, meningeal layers, and its surrounding cerebrospinal fluid (CSF) in both humans and rodents. While neuro-immune interactions have been classically studied in the context of neuroinflammatory disease, recent works have also elucidated unconventional roles of immune-derived cytokines in neurological function, highlighting the many implications and potential of neuro-immune interactions. As a result, the study of neuro-immune interactions is becoming increasingly important in understanding both CNS homeostasis and disease. Here, we review the anatomically distinct immune compartments within the brain, the known mechanisms of leukocyte trafficking and infiltration into the CNS and unique transcriptional and functional characteristics of CNS-resident immune cells.


Similar content being viewed by others
References
Rua R, McGavern DB (2018) Advances in meningeal immunity. Trends Mol Med 24(6):542–559
Li Q, Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18(4):225–242
Mascagni, and C. Sanctius, 1787 Vasorum lymphaticorum corporis humani historia et ichnographia. Ex typographia Pazzini Carli.
Andres KH et al (1987) Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 175(3):289–301
Waggener JD, Beggs J (1967) The membranous coverings of neural tissues: an electron microscopy study. J Neuropathol Exp Neurol 26(3):412–426
Louveau A et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Aspelund A et al (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212(7):991–999
Absinta, M., et al (2017) Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife, 6.
Mundt S et al (2019) The CNS Immune Landscape from the Viewpoint of a T Cell. Trends Neurosci 42(10):667–679
Protasoni M et al (2011) The collagenic architecture of human dura mater. J Neurosurg 114(6):1723–1730
Schafflick D et al (2021) Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci 24(9):1225–1234
Korin B et al (2017) High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci 20(9):1300–1309
Mrdjen D et al (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48(2):380-395.e6
Roth TL et al (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505(7482):223–228
Hagan CE, Bolon B, Keene CD (2012) 20 - Nervous system. In: Treuting M, Dintzis SM (eds) Comparative anatomy and histology. Academic Press, San Diego, pp 339–394
Iliff JJ (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes including amyloid β. Sci Transl Med 4(147):147ra111
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17(11):1016–1024
Brinker T et al (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10
McComb JG (1983) Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg 59(3):369–383
Hafler DA et al (1985) In vivo activated T lymphocytes in the peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. N Engl J Med 312(22):1405–1411
de Graaf MT et al (2011) Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom 80(1):43–50
Svenningsson A et al (1995) Lymphocyte phenotype and subset distribution in normal cerebrospinal fluid. J Neuroimmunol 63(1):39–46
Kowarik MC et al (2014) Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol 261(1):130–143
Lepennetier G et al (2019) Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J Neuroinflammation 16(1):219
Gate D et al (2020) Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577(7790):399–404
Wang, et al (2021) Single-cell transcriptome and TCR profiling reveal activated and expanded T cell populations in Parkinson’s disease. Cell Discov. 7(1):52
Ballabh A Braun, Nedergaard M (2004) The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16(1):1–13
Smolders J et al (2018) Tissue-resident memory T cells populate the human brain. Nat Commun 9(1):4593
Pasciuto E et al (2020) Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell 182(3):625-640.e24
Lopes Pinheiro MA et al (2016) Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta 1862(3):461–471
Stern JN et al (2014) B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med 6(248):248ra107
Brioschi S et al (2021) Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 373(6553)
Unger MS et al (2020) CD8+ T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav Immun 89:67–86
Williams G et al (2021) CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 144(7):2047–2059
Gate, D et al. 2021 CD4<sup>+</sup> T cells contribute to neurodegeneration in Lewy body dementia. Science. 0(0): eabf7266.
Bitsch A et al (2000) Acute axonal injury in multiple sclerosis. Correlation demyelination inflamm Brain 123(Pt 6):1174–1183
Prinz M, Priller J (2010) Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol 224(1–2):80–84
Jordão MJC et al (2019) Single cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363:6425
Auffray C et al (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317(5838):666–670
Fife BT et al (2000) CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med 192(6):899–905
Huang D et al (2001) Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 193(6):713–726
Boring L et al (1997) Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 100(10):2552–2561
Peters W et al (2001) Chemokine receptor 2 serves an early and essential role in resistance to <em>Mycobacterium tuberculosis</em>. Proc Natl Acad Sci 98(14):7958–7963
Ajami B et al (2011) Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14(9):1142–1149
King IL, Dickendesher TL, Segal BM (2009) Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 113(14):3190–3197
Amorim A et al (2022) IFNγ and GM-CSF control complementary differentiation programs in the monocyte-to-phagocyte transition during neuroinflammation. Nat Immunol 23(2):217–228
Spiljar M et al (2021) Cold exposure protects from neuroinflammation through immunologic reprogramming. Cell Metab 33(11):2231-2246.e8
Herisson F et al (2018) Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci 21(9):1209–1217
Cugurra A et al (2021) Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science 373(6553)
Ahn JJ, Abu-Rub M, Miller RH (2021) B Cells in neuroinflammation: new perspectives and mechanistic insights. Cells 10:7
Franciotta D et al (2008) B cells and multiple sclerosis. Lancet Neurol 7(9):852–858
Barr TA et al (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6–producing B cells. J Exp Med 209(5):1001–1010
Matsushita T et al (2008) Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Investig 118(10):3420–3430
Weber MS et al (2010) B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol 68(3):369–383
Pierson ER, Stromnes IM, Governan JM (2014) B cells promote induction of experimental autoimmune encephalomyelitis by facilitating reactivation of T cells in the central nervous system. J immunol (Baltimore Md 1950) 192(3):929–939
Duddy M et al (2007) Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 178(10):6092–6099
Bar-Or A et al (2010) Abnormal B-cell cytokine responses a trigger of T-cell–mediated disease in MS? Ann Neurol 67(4):452–461
Hafler DA et al (1985) Investigation of in vivo activated T cells in multiple sclerosis and inflammatory central nervous system diseases. Clin Immunol Immunopathol 37(2):163–171
Hafler DA, Weiner HL (1987) In vivo labeling of blood T cells: rapid traffic into cerebrospinal fluid in multiple sclerosis. Ann Neurol 22(1):89–93
Mahad D et al (2006) Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain 129(Pt 1):212–223
Balashov KE et al (1999) CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A 96(12):6873–6878
Kivisäkk, et al (2002) T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol 129(3):510–8
Reboldi A et al (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10(5):514–523
Pappalardo JL et al (2020) Transcriptomic and clonal characterization of T cells in the human central nervous system. Sci Immunol 5:eabb8786
Chofflon M et al (1989) Inflammatory cerebrospinal fluid T cells have activation requirements characteristic of CD4+CD45RA- T cells. Eur J Immunol 19(10):1791–1795
Kivisäkk, et al (2003) Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A 100(14):8389–94
Van Hove H et al (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22(6):1021–1035
Ginhoux F et al (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845
Utz SG et al (2020) Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell 181(3):557-573.e18
Frost JL, Schafer D (2016) Microglia: architects of the developing nervous system. Trends Cell Biol 26(8):587–597
Schafer D, Stevens B (2015) Microglia function in central nervous system development and plasticity. Cold Spring Harb Perspect Biol 7(10):a020545
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318
Prinz M et al (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14(10):1227–1235
Ueno M et al (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16(5):543–551
Arcuri C et al (2019) Parenchymal and non-parenchymal immune cells in the brain: a critical role in regulating CNS functions. Int J Dev Neurosci 77:26–38
Marín-Teva JL et al (2004) Microglia promote the death of developing Purkinje cells. Neuron 41(4):535–547
Zhan Y et al (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17(3):400–406
Schafer D et al (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74(4):691–705
Paolicelli RC et al (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048):1456–1458
Cunningham CL, Martínez-Cerdeño V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33(10):4216–4233
Sierra A et al (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7(4):483–495
Frade JM, Barde YA (1998) Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron 20(1):35–41
Battista D et al (2006) Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci 23(1):83–93
Dong Y, Yong VW (2019) When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol 15(12):704–717
Goldmann T et al (2016) Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 17(7):797–805
Anthony IC, Crawford DH, Bell JE (2003) B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain 126(Pt 5):1058–1067
Berer K et al (2011) Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479(7374):538–541
Fitzpatrick Z et al (2020) Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 587(7834):472–476
Pröbstel AK et al (2020) Gut microbiota-specific IgA(+) B cells traffic to the CNS in active multiple sclerosis. Sci Immunol 5(53)
Rattazzi L et al (2013) CD4+ but not CD8+ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiatry 3(7):e280–e280
Fan K-Q et al (2019) Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell 179(4):864-879.e19
Yonk LJ et al (1990) CD4+ helper T cell depression in autism. Immunol Lett 25(4):341–345
Marazziti D et al (1999) Immunological alterations in adult obsessive-compulsive disorder. Biol Psychiat 46(6):810–814
Miyajima M et al (2017) Metabolic shift induced by systemic activation of T cells in PD-1-deficient mice perturbs brain monoamines and emotional behavior. Nat Immunol 18(12):1342–1352
Derecki NC et al (2010) Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med 207(5):1067–1080
Filiano AJ et al (2016) Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535(7612):425–429
Alves de Lima K et al (2020) Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol 21(11):1421–1429
Ribeiro M et al (2019) Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short term memory. Sci Immunol 4(40)
Clark IC et al (2021) Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372(6540):eabf1230
Funding
This study was supported by grants to D. A. H. from the National Institutes of Health (NIH) (U19 AI089992, R25 NS079193, P01 AI073748, U24 AI11867, R01 AI22220, UM 1HG009390, P01 AI039671, P50 CA121974, and R01 CA227473); the National Multiple Sclerosis Society (NMSS) (CA 1061-A-18 and RG-1802–30153); the Nancy Taylor Foundation for Chronic Diseases, and Erase MS, by grants to. A. W. from the NIH (K08 AI128745, and R01 AI162645); the Pew Charitable Trusts; and the Richard and Susan Smith Family Foundation, and by grants to T. Y. from the NIH (5T32 AI 007019–46).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. A. H. has received research funding from Bristol-Myers Squibb, Novartis, Sanofi, and Genentech. He has been a consultant for Bayer Pharmaceuticals, Bristol Myers Squibb, Compass Therapeutics, EMD Serono, Genentech, Juno therapeutics, Novartis Pharmaceuticals, Proclara Biosciences, Sage Therapeutics, and Sanofi Genzyme. Further information regarding funding is available on https://openpaymentsdata.cms.gov/physician/166753/general-payments
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on: Neuroimmune Interactions in Health and Disease - Guest Editors: David Hafler & Lauren Sansing
Rights and permissions
About this article
Cite this article
Yoshida, T.M., Wang, A. & Hafler, D.A. Basic principles of neuroimmunology. Semin Immunopathol 44, 685–695 (2022). https://doi.org/10.1007/s00281-022-00951-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-022-00951-7