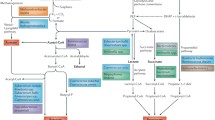
Abstract
The human intestine is believed to contain approximately 100 trillion intestinal (gut) microbiota, comprising about 500–1000 different species. These intestinal microbiota exist in a symbiotic relationship with their host, by metabolizing compounds that the host is unable to utilize and controlling the immune balance of the host’s body. However, the composition of the intestinal microbiota is known to vary, depending on diet, nutrition status, and other factors. The recently developed meta-omics microbial data and the technical progress for the metabolome analysis provide a substantial understanding of the role of intestinal microbes and their metabolism. Interestingly, accumulating evidence suggests that the intestinal microbiota contributes to the onset of colorectal cancer, not only via the pro-carcinogenic activities of specific pathogens but also via the influence of the bacterial metabolites. Moreover, since the gut microbial metabolites circulate in the host’s body, it has been increasingly recognized that the intestinal microbiota are involved in the pathogenesis of diseases not only in the intestine but also in the organs located distant from the intestine. We recently found that metabolites from obesity-induced intestinal microbiota promoted liver cancer, and elucidated the underlying molecular mechanism. In this review, I first summarize the general understanding on the carcinogenic process by bacterial metabolites, and then discuss on the association between intestinal microbiota and colorectal cancer. In the last part, I will introduce our recent findings on liver cancer promotion by a metabolite of the obesity-induced intestinal microbiota.


Similar content being viewed by others
References
Kamada N, Seo SU, Chen GY et al (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13:321–335
Ley RE, Turnbaugh PJ, Klein S et al (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023
Ley RE, Bäckhed F, Turnbaugh P et al (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102:11070–11075
Holmes E, Li JV, Marchesi JR et al (2012) Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 16:559–564
Fritz JV, Desai MS, Shah P et al (2013) From meta-omics to causality: experimental models for human microbiome research. Microbiomedicine 1:14
Ou J, Carbonero F, Zoetendal EG et al (2013) Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 98:111–120
Wu S, Rhee KJ, Albesiano E et al (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15:1016–1022
Arthur JC, Perez-Chanona E, Mühlbauer M et al (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338:120–123
Louis P, Hold GL, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12:661–672
Gonçalves P, Martel F (2013) Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab 14:994–1008
Henao-Mejia J, Elinav E, Jin C et al (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482:179–185
Koeth RA, Wang Z, Levison BS et al (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585
Yokote H, Miyake S, Croxford JL et al (2008) NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am J Pathol 173:1714–1723
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10:735–742
Yoshimoto S, Loo TM, Atarashi K et al (2013) Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499:97–101
Natarajan N, Pluznick JL (2014) From microbe to man: the role of microbial short chain fatty acid metabolites in host cell biology. Am J Physiol Cell Physiol. doi:10.1152/ajpcell.00228.2014
Sleeth ML, Thompson EL, Ford HE et al (2010) Free fatty acid receptor 2 and nutrient sensing: a proposed role for fibre, fermentable carbohydrates and short-chain fatty acids in appetite regulation. Nutr Res Rev 23:135–145
Fung KYV, Cosgrove L, Lockett T et al (2012) A review of the potential mechanism for the lowering of colorectal oncogenesis by butyrate. Br J Nutr 108:820–831
Hamer HM, Jonkers D, Venema K et al (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–119
Chang PV, Hao L, Offermanns S et al (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111:2247–2252
Smith PM, Howitt MR, Panikov N et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 341:569–573
Furusawa Y, Obata Y, Fukuda S et al (2013) Commensal microbe-derived butyrate induced the differentiation of colonic regulatory T cells. Nature 504:446–450
Arpaia N, Campbell C, Fan X et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455
Singh N, Gurav A, Sivaprakasam S et al (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139
Ridlon JM, Kang DJ, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259
Zhou H, Hylemon PB (2014) Bile acids are nutrient signaling hormones. Steroids 86C:62–68
Kitazawa S, Denda A, Tsutsumi M et al (1990) Enhanced preneoplastic liver lesion development under ‘selection pressure’ conditions after administration of deoxycholic or lithocholic acid in the initiation phase in rats. Carcinogenesis 11:1323–1328
Ridlon JM, Hylemon PB (2012) Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7a-dehydroxylating intestinal bacterium. J Lipid Res 53:66–76
Payne CM, Weber C, Crowley-Skillicorn C et al (2007) Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis 28:215–2
Reddy BS, Wynder EL (1973) Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst 50:1437–1442
Pai R, Tarnawski AS, Tran T (2004) Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol Biol Cell 15:2156–2163
d’Adda di Fagagna F (2008) Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 8:512–522
Kuilman T, Michaloglou C, Mooi WJ et al (2010) The essence of senescence. Genes Dev 24:2463–2479
Ohtani N, Hara E (2013) Roles and mechanisms of cellular senescence in regulation of tissue homeostasis. Cancer Sci 104:525–530
Coppé JP, Patil CK, Rodier F et al (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868
Rodier F, Coppé JP, Patil CK et al (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11:973–979
Takahashi A, Imai Y, Yamakoshi K et al (2012) DNA damage signaling triggers degradation of histone methyltransferases through APC/C(Cdh1) in senescent cells. Mol Cell 45:123–131
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192:547–556
Kuilman T, Peeper DS (2009) Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9:81–94
Acosta JC, O’Loghlen A, Banito A et al (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133:1006–1018
Kuilman T, Michaloglou C, Vredeveld LC et al (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133:1019–1031
Kortlever RM, Higgins PJ, Bernards R (2006) Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8:877–884
Sparmann A, Bar-Sagi D (2004) Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6:447–458
Ancrile B, Lim KH, Counter CM (2007) Oncogenic ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev 21:1714–1719
Park EJ, Lee JH, Yu GY et al (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140:197–208
Ohanna M, Giuliano S, Bonet C et al (2011) Senescent cells develop a PARP-1 and nuclear factor-kB-associated secretome (PNAS). Genes Dev 25:1245–1261
Yang G, Rosen DG, Zhang Z et al (2006) The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci U S A 103:16472–16477
Cuevas-Ramos G, Petit CR, Marcq I et al (2010) Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A 107:11537–11542
Cougnoux A, Dalmasso G, Martinez R et al (2014) Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut. doi:10.1136/gutjnl-2013-305257
Castellarin M, Warren RL, Freeman JD et al (2012) Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22:299–306
Kostic AD, Gevers D, Pedamallu CS et al (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22:292–29
Kostic AD, Chun E, Robertson L et al (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14:207–215
Rubinstein MR, Wang X, Liu W et al (2013) Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14:195–206
Homann N (2001) Alcohol and upper gastrointestinal tract cancer: the role of local acetaldehyde production. Addict Biol 6:309–323
Bode C, Bode JC (2005) Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res 29:166S–171S
Khandekar MJ, Cohen P, Spiegelman BM (2011) Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 11:886–895
Jais A, Einwallner E, Sharif O et al (2014) Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 158:25–40
Calle EE, Rodriguez C, Walker-Thurmond K et al (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625–1638
Samanic C, Gridley G, Chow WH et al (2004) Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 15:35–43
Møller H, Mellemgaard A, Lindvig K et al (1994) Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer 30A:344–350
Wolk A, Gridley G, Svensson M et al (2001) A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 12:13–21
Haslam DW, James WP (2005) Obes Lancet 366(9492):1197–1209
Ohtani N, Imamura Y, Yamakoshi K et al (2007) Visualizing the dynamics of p21(Waf1/Cip1) cyclin-dependent kinase inhibitor expression in living animals. Proc Natl Acad Sci U S A 104:15034–15039
Sato Y, Murase K, Kato J et al (2008) Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol 26:431–442
Dumas ME, Barton RH, Toye A et al (2006) Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A 103:12511–12516
Ridaura VK, Faith JJ, Rey FE et al (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214
Dapito DH, Mencin A, Gwak GY et al (2012) Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21:504–516
Minamida K, Ohashi M, Hara H et al (2006) Effects of ingestion of difructose anhydride III (DFA III) and the DFA III-assimilating bacterium Ruminococcus productus on rat intestine. Biosci Biotechnol Biochem 70:332–339
Beuers U (2006) Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol 3:318–328
Takuma Y, Nouso K (2010) Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol 16:1436–1441
Rafter JJ, Child P, Anderson AM et al (1987) Cellular toxicity of fecal water depends on diet. Am J Clin Nutr 45:559–563
David LA, Maurice CF, Carmody RN et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the Special Issue on Microbiome, Immunity and Inflammation - Guest Editor: Hiroshi Ohno
Rights and permissions
About this article
Cite this article
Ohtani, N. Microbiome and cancer. Semin Immunopathol 37, 65–72 (2015). https://doi.org/10.1007/s00281-014-0457-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-014-0457-1